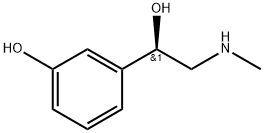
3-Hydroxy-alpha-((methylamino)-methyl)benzolmethanol, (R)-
|
|
3-Hydroxy-alpha-((methylamino)-methyl)benzolmethanol, (R)- Eigenschaften
- Schmelzpunkt:
- 171°C
- Siedepunkt:
- 295.79°C (rough estimate)
- Dichte
- 1.1222 (rough estimate)
- Brechungsindex
- -55.5 ° (C=5, 1mol/L HCl)
- storage temp.
- -20°C Freezer, Under inert atmosphere
- L?slichkeit
- Slightly soluble in water, sparingly soluble in methanol, slightly soluble in ethanol (96 per cent). It dissolves in dilute mineral acids and in dilute solutions of alkali hydroxides.
- pka
- pKa 8.9 (Uncertain)
- Aggregatzustand
- Solid
- Farbe
- White to Off-White
- CAS Datenbank
- 59-42-7(CAS DataBase Reference)
- NIST chemische Informationen
- Benzenemethanol, 3-hydroxy-«alpha»-[(methylamino)methyl]-, (r)-(59-42-7)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| Kennzeichnung gef?hrlicher | Xn | ||
|---|---|---|---|
| R-S?tze: | 22-36/37/38-41-37/38 | ||
| S-S?tze: | 26-36-45-39 | ||
| RTECS-Nr. | DO7175000 | ||
| HS Code | 2922.19.7000 | ||
| Giftige Stoffe Daten | 59-42-7(Hazardous Substances Data) | ||
| Toxizit?t | LD50 oral in rat: 350mg/kg |
| Bildanzeige (GHS) |
 
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Achtung | |||||||||||||||||||||||||||||||||||
| Gefahrenhinweise |
|
|||||||||||||||||||||||||||||||||||
| Sicherheit |
|
3-Hydroxy-alpha-((methylamino)-methyl)benzolmethanol, (R)- Chemische Eigenschaften,Einsatz,Produktion Methoden
R-S?tze Betriebsanweisung:
R22:Gesundheitssch?dlich beim Verschlucken.R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
S-S?tze Betriebsanweisung:
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
S45:Bei Unfall oder Unwohlsein sofort Arzt zuziehen (wenn m?glich, dieses Etikett vorzeigen).
Beschreibung
This synthetic drug has both chemical and pharmacological similarities to norepinephrine. A characteristic quality of phenylephrine is the distinctly expressed selectivity to α- adrenoreceptors, especially α1-adrenoreceptors. Although phenylephrine increases the contractibility of blood vessels, in practical terms it is not considered a cardiostimulant.Chemische Eigenschaften
White or almost white, crystalline powder.Verwenden
L-Phenylephrine is an adrenergic α1A receptor agonist (Ki = 1.4 μM) that demonstrates selectivity against the α1B and α1C receptor subtypes (Kis = 23.9 and 47.8 μM, respectively). By stimulating adrenergic α1 receptors, L-phenylephrine can induce aortic smooth muscle contractions, although reported relative affinity and potency values in rabbit are 5-fold weaker compared to that of L-norepinephrine. This compound is frequently used to precontract smooth muscle in preparations designed to study the properties of various vasodilator agents. Because L-phenylephrine acts on adrenergic α1 receptors in the arterioles of the nasal mucosa to produce constriction, it has been examined clinically as an oral decongestant.Definition
ChEBI: A member of the class of the class of phenylethanolamines that is (1R)-2-(methylamino)-1-phenylethan-1-ol carrying an additional hydroxy substituent at position 3 on the phenyl ring.Allgemeine Beschreibung
(Neo-Synephrine, a prototypical selectivedirect-acting 1-agonist) differs from E only inlacking a p-OH group. It is orally active, and its DOA isabout twice that of E because it lacks the catechol moietyand thus is not metabolized by COMT. However, its oralbioavailability is less than 10% because of its hydrophilicproperties (log P=0.3), intestinal 3 -O-glucuronidation/sulfation and metabolism by MAO. Lacking the p-OHgroup, it is less potent than E and NE but it is a selectiveα1-agonist and thus a potent vasoconstrictor. It is usedsimilarly to metaraminol and methoxamine for hypotension.Another use is in the treatment of severe hypotensionresulting from either shock or drug administration. It alsohas widespread use as a nonprescription nasal decongestantin both oral and topical preparations. When applied tomucous membranes, it reduces congestion and swelling byconstricting the blood vessels of the membranes. In theeye, it is used to dilate the pupil and to treat open-angleglaucoma. In addition, it is used in spinal anesthesia toprolong the anesthesia and to prevent a drop in blood pressureduring the procedure. It is relatively nontoxic and produceslittle CNS stimulation. Metaraminol is just anotherexample.Clinical Use
Phenylephrine is a potent direct-acting α1-agonist with clinical effects similar to those of noradrenaline. It causes widespread vasoconstriction with an increase in arterial pressure, reflex bradycardia and decrease in cardiac output. It may be administered by i.v. bolus (50–100μg boluses) and i.v. infusion (50–150μgmin –1) to maintain arterial pressure during general anaesthesia or other causes of low SVR. It may also be used topically as a nasal decongestant or mydriatic. There is some evidence suggesting a paradoxical reduction in cerebral oxygen delivery.Sicherheitsprofil
Poison by ingestion, subcutaneous, intravenous, intraperitoneal, and intraduodenal routes. Human systemic effects by ocular route: blood pressure increase. An experimental teratogen. Other experimental reproductive effects. A nasal decongestant. When heated to decomposition it emits toxic fumes of NOx.3-Hydroxy-alpha-((methylamino)-methyl)benzolmethanol, (R)- Upstream-Materialien And Downstream Produkte
Upstream-Materialien
(±)-3-Hydroxy-α-[(methylamino)methyl]benzylalkohol
(S)-3-Hydroxy-α-[(methylamino)methyl]benzylalkohol
Benzenemethanol, 3-methoxy-α-[(methylamino)methyl]-, (αR)-
3-Methoxybenzaldehyd
1-(3-Hydroxyphenyl)-2-(methylamino)ethanone hydrochloride
Downstream Produkte
3-Hydroxy-alpha-((methylamino)-methyl)benzolmethanol, (R)- Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 202)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| Shanghai Worldyang Chemical Co.,Ltd. | +86-021-56795779 +8613651600618 |
sales@worldyachem.com | China | 1243 | 58 |
| Hangzhou FandaChem Co.,Ltd. | +8615858145714 |
FandaChem@Gmail.com | China | 9087 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29811 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569266 15319487004 |
1015@dideu.com | China | 3942 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 32161 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 |
sales@tnjchem.com | China | 34563 | 58 |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 |
sales@afinechem.com | China | 15352 | 58 |
| Alfa Chemistry | |
info@alfa-chemistry.com | United States | 2344 | 58 |
| Wuhan Fortuna Chemical Co., Ltd | +86-027-59207850 |
info@fortunachem.com | China | 5975 | 58 |
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 |
dominicguo@gk-bio.com | CHINA | 9414 | 58 |
59-42-7(3-Hydroxy-alpha-((methylamino)-methyl)benzolmethanol, (R)-)Verwandte Suche:
Methyl-4-hydroxybenzoat
Trometamol
3-Hydroxy-alpha-((methylamino)-methyl)benzolethanol-hydro-chlorid, (R)
α-Chlor-o-xylol
(±)-3-Hydroxy-α-[(methylamino)methyl]benzylalkoholhydrochlorid
Chenodesoxycholsure
3,6-Bis[(4-chlor-2-phosphonophenyl)azo]-4,5-dihydroxynaphthalin-2,7-disulfonsure
Benzylalkohol
Methylacrylat
2-Phenylethanol
N,N-Dimethyl-phenylendiamin (p)
Doxifluridin
Ursodeoxycholsure
Bensulfuron methyl
Kresoxim-methyl
Procarbazin
Methyl
Brommethan
- component of Dristan cold
- component of Dristan nasal mist
- component of Entex
- component of Histalet forte
- component of Hycomine compound
- component of Prefrin-a
- component of Pv tussin syrup
- component of Relief
- (-)-PHENYLEPHRINE
- (-)-m-Hydroxy-alpha-(methylaminomethyl)benzyl alcohol
- component of Ru-tuss liquid
- component of Ru-tuss tablets
- component of Vasosulf
- component of Zincfrin
- Isophrin
- l-(3-Hydroxyphenyl)-N-methylethanolamine
- l-1-(m-Hydroxyphenyl)-2-methylaminoethanol
- l-alpha-Hydroxy-beta-methylamino-3-hydroxy-l-ethylbenzene
- l-m-Hydroxy-alpha-((methylamino)methyl)benzyl alcohol
- l-m-hydroxy-alpha-((methylamino)methyl)benzylalcohol
- Mesaton
- Mesatone
- Metaoxedrin
- Metaoxedrine
- Metasympatol
- Metasynephrine
- Mezaton
- m-Methylaminoethanolphenol
- m-Oxedrine
- m-Sympathol
- m-Sympatol
- m-Synephrine
- Mydfrin
- Neophryn
- Neosynephrine
- Nostril
- Prefrin
- Pyracort D
- R(-)-Mezaton
- R(-)-Phenylephrine
- Visadron
- 3-(1-HYDROXY-2-METHYLAMINO-ETHYL)PHENOL
- 7: PN: US20030109453 SEQID: 6 claimed sequence
- Benzenemethanol, 3-hydroxy-a-[(methylamino)methyl]-, (aR)- (9CI)
- Benzenemethanol, 3-hydroxy-a-[(methylamino)methyl]-, (R)-
- l-m-Hydroxy-a-[(methylamino)methyl]benzyl alcohol
- L-Phenylephedrine
- Phenylephrine (base and/or unspecified salts)
- 3-Hydroxy-alpha-((methylamino)methyl)-benzyl alcohol
- α-[(Methylamino)methyl]-3-hydroxybenzenemethanol
- (-)-1-(3-hydroxyphenyl)-2-methylaminoethanol
- C07441
- BenzeneMethanol,3-hydroxy-a-[(MethylaMino)Methyl]-, (aR)-
- L-Phenylephrine
- R-(γ)-Phe
- PHARMA CHEMICAL PHENYLEPHRINE HCL
- (-)-m-hydroxy-alpha-(methylaminomethyl)benzylalcohol
- (-)-m-oxedrine