
Oseltamivir phosphate synthesis
- Product Name:Oseltamivir phosphate
- CAS Number:204255-11-8
- Molecular formula:C16H31N2O8P
- Molecular Weight:410.4
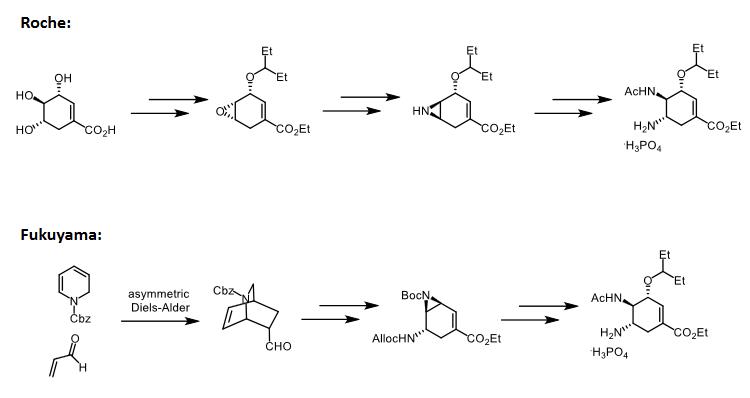
It can also be obtained by a novel 12-step synthesis from (-)-quinic acid.
196618-13-0
213 suppliers
inquiry

204255-11-8
552 suppliers
$9.00/25mg
Yield:204255-11-8 98%
Reaction Conditions:
with phosphoric acid in ethanol at 50; for 0.0166667 h;Sonication;
Steps:
8 Reaction 8: Continuous flow synthesis of Oseltamivir Phosphate 3
Oseltamivir 33 was treated with FI2PO4 to afford Oseltamivir phosphate 3 (Scheme 18) in a continuous flow system. Scheme 19 (0204) A 0.8 ml PTFE coil reactor (0.8 mm ID, 1 .6 m tube length) under sonication was used to optimise the treatment of Oseltamivir 33 with H2PO4 affording Oseltamivir phosphate 3 in a continuous flow system. Oseltamivir 33 (0.1 M) in ethanol and H2PO4 (0.12 M, 1 .2 equiv.) in ethanol were pumped through a thermally controlled continuous flow system to afford oseltamivir phosphate. Samples were collected and analysed using HPLC method A. (0205) Oseltamivir 33 (0.1 M) in ethanol was treated with H2PO4 (0.12 M, 1 .2 equiv.) in ethanol at 50 °C in a 0.8 ml PTFE coil reactor (0.8 mm ID, 1 .6 m tube length) under sonication at various residence times for optimisation. The results of these experiments are shown in Figure 20. (0206) Conversion to Oseltamivir phosphate 3 increased with increase in residence time. A conversion plateau of 98 % (FIPLC) towards Oseltamivir phosphate 3 was reached at 60 s residence time. Based on these experiments, the preferred conditions were about 50 °C and 60 s residence time to give Oseltamivir phosphate 3 (98 %, FIPLC), which is a significant improvement an any previously reported reaction. (0207) This above description of some of the illustrative embodiments of the invention is to indicate how the invention can be made and carried out. Those of ordinary skill in the art will know that various details may be modified thereby arriving at further embodiments, but that many of these embodiments will remain within the scope of the invention.
References:
WO2020/183281,2020,A1 Location in patent:Page/Page column 7; 33; 34
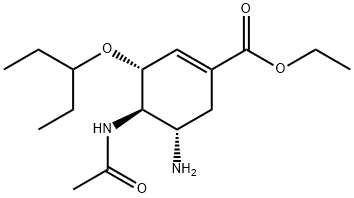
1041262-68-3
0 suppliers
inquiry

204255-11-8
552 suppliers
$9.00/25mg

204255-06-1
92 suppliers
inquiry

204255-11-8
552 suppliers
$9.00/25mg
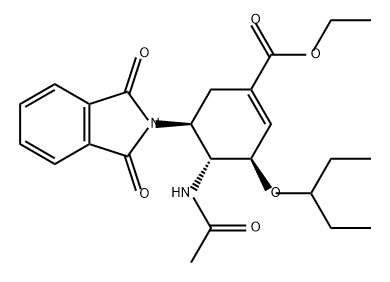
367252-68-4
1 suppliers
inquiry

204255-11-8
552 suppliers
$9.00/25mg
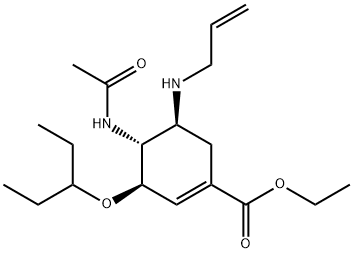
312904-18-0
16 suppliers
inquiry

204255-11-8
552 suppliers
$9.00/25mg
