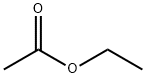
Ethyl acetate synthesis
- Product Name:Ethyl acetate
- CAS Number:141-78-6
- Molecular formula:C4H8O2
- Molecular Weight:88.11
Ethyl acetate is a colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, and in the decaffeination process of tea and coffee.
Ethyl acetate is synthesized in industry mainly via the classic Fischer esterification reaction of ethanol and acetic acid. This mixture converts to the ester in about 65% yield at room temperature:
CH3CO2H + CH3CH2OH → CH3CO2CH2CH3 + H2O
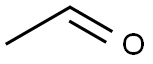
75-07-0
422 suppliers
$14.00/5mL

141-78-6
633 suppliers
$22.37/250ml
Yield:141-78-6 95%
Reaction Conditions:
with [{(PhN)MeC(Nt-Bu)}AlMe(μ-OMe)]2 at 20; for 16 h;Inert atmosphere;Schlenk technique;Green chemistry;Tishchenko-Claisen Dismutation;
Steps:
2.5 General procedure employed for the Tishchenko reaction
General procedure: The pre-catalyst, amidinatoaluminum compound (0.8 mmol) was placed in a dry Schlenk flask under a nitrogen atmosphere, and freshly distilled aldehyde (80 mmol) was introduced. The mixture was immediately stirred at room temperature for 30 min to produce the corresponding ester. The reaction was quenched with 0.5 ml of water and the product was distilled to collect the corresponding ester. The yields reported are the isolated yield.
References:
Zhang, Shaofeng;Han, Hongfei;Guo, Zhiqiang;Tong, Hongbo;Wei, Xuehong [Polyhedron,2015,vol. 90,p. 118 - 122]

64-17-5
727 suppliers
$10.00/50g
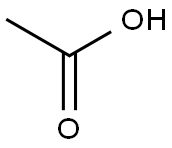
64-19-7
1563 suppliers
$10.00/25ML

141-78-6
633 suppliers
$22.37/250ml

64-17-5
727 suppliers
$10.00/50g

75-07-0
422 suppliers
$14.00/5mL

64-19-7
1563 suppliers
$10.00/25ML

141-78-6
633 suppliers
$22.37/250ml

64-17-5
727 suppliers
$10.00/50g

75-07-0
422 suppliers
$14.00/5mL

141-78-6
633 suppliers
$22.37/250ml