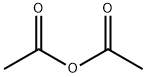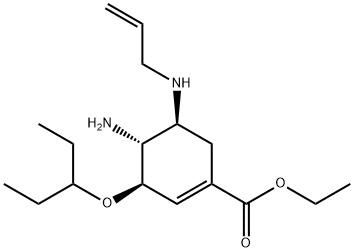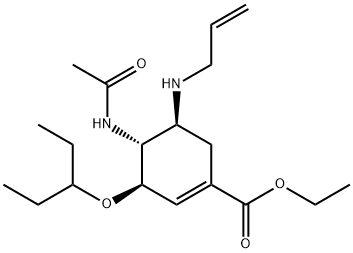
312904-18-0 synthesis
- Product Name:312904-18-0
- CAS Number:312904-18-0
- Molecular formula:C19H32N2O4
- Molecular Weight:352.47
Yield:312904-18-0 83%
Reaction Conditions:
with methanesulfonic acid;acetic acid in tert-butyl methyl ether;water at 0 - 23; for 14.6 h;
Steps:
1.e
In a 414-necked round bottom flask equipped with a thermometer, a mechanical stirrer, a Claisen condenser and an inert gas supply 278.0 g of (3R,4R,5S)-5-allylamino-4-amino-3-(1-ethyl-propoxy)-cyclohex-1-enecarboxylic acid ethyl ester obtained according to (d) were dissolved at room temperature with stirring under argon in 2800 ml of tert.-butyl methyl ether. From the red solution 1400 ml of tert.-butyl methyl ether were distilled. Again 1400 ml of tert.-butyl methyl ether were added and distilled off. The red solution was cooled to 0-5° C. and treated with 512 ml of acetic acid (9.0 mol) whereby the temperature rose to about 23° C. After cooling to 0° C.-5° C. 58.1 ml of methanesulfonic acid (d=1.482, 0.90 mol) were added dropwise in the course of 27 min followed by 84.7 ml of acetic anhydride (d=1.08, 0.90 mol) added dropwise in the course of 40 min keeping the temperature in the range of 0° C. to 5C. The brown reaction mixture was stirred without cooling for 14 h then treated with vigorous stirring with 1400 ml of water (deionized) for 30 min and the brown organic phase was extracted with 450 ml of 1M aqueous methanesulfonic acid. The combined aqueous phases (pH=1.6) were treated with stirring with about 694 ml of 50% aqueous potassium hydroxide until pH=10.0 was reached, keeping the temperature in the range of 10 to 25° C. The brown, turbid mixture was extracted first with 1000 ml then with 400 ml, in total with 1400 ml of tert.-butyl methyl ether, the combined organic extracts were stirred over 32 g of charcoal and filtered. The filter cake was washed with about 200 ml tert.-butyl methyl ether and the combined filtrates were evaporated in a rotary evaporator at 47° C./380 to 10 mbar to yield 285.4 g of brown-red, amorphous crystals which were dissolved with stirring in a mixture of 570 ml of tert.-butyl methyl ether and 285 ml of n-hexane at 50° C. The brown solution was cooled in 45 min with stirring to -20° C. to -25° C. and stirred for 5 h whereby brown crystals precipitated. The suspension was filtered over a pre-cooled (-20° C.) glass filter funnel and the filter cake was washed with a pre-cooled (-20° C.) mixture of 285 ml of tert.-butyl methyl ether and 143 ml of n-hexane and dried in a rotary evaporator at 48° C.<10 mbar to yield 200.33 g (83%) of (3R,4R,5S)-4-acetylamino-5-allylamino-3-(1-ethyl-propoxy)-cyclohex-1-enecarboxylic acid ethyl ester;
References:
US2006/47002,2006,A1 Location in patent:Page/Page column 7
![(1S,5R,6S)-Ethyl 5-(pentan-3-yl-oxy)-7-oxa-bicyclo[4.1.0]hept-3-ene-3-carboxylate](/CAS/GIF/204254-96-6.gif)
204254-96-6
304 suppliers
$85.00/500mg

312904-18-0
15 suppliers
inquiry
312904-12-4
4 suppliers
inquiry

312904-18-0
15 suppliers
inquiry
332047-22-0
0 suppliers
inquiry

312904-18-0
15 suppliers
inquiry
![1-Cyclohexene-1-carboxylic acid, 5-amino-3-(1-ethylpropoxy)-4-[(methylsulfonyl)oxy]-, ethyl ester, (3R,4S,5R)-](/CAS/20210305/GIF/312904-17-9.gif)
312904-17-9
1 suppliers
inquiry

312904-18-0
15 suppliers
inquiry