Oseltamivir synthesis
- Product Name:Oseltamivir
- CAS Number:196618-13-0
- Molecular formula:C16H28N2O4
- Molecular Weight:312.4

204255-11-8
539 suppliers
$9.00/25mg

196618-13-0
213 suppliers
inquiry
Yield:196618-13-0 98%
Reaction Conditions:
with sodium hydrogencarbonate in water at 20; for 0.0833333 h;Inert atmosphere;
Steps:
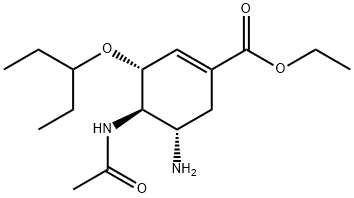
3 4.1.3 Ethyl (3R,4R,5S)-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate (3)
To a solution of oseltamivir phosphate (1.0 g, 2.43 mmol) in water (30 mL) was added a saturated solution of sodium bicarbonate (10 mL).
The reaction mixture was stirred for 5 min at room temperature.
The mixture was extracted with DCM/MeOH mixture (3:1; 4 * 10 mL).
The combined organic phase was washed with brine, dried over anhydrous Na2SO4 and concentrated under reduced pressure to furnish the free base of oseltamivir (0.75 g, 98% yield).
The product was used without further purification. 1H NMR (300 MHz, CDCl3) δ 6.78 (t, J = 2.1 Hz, 1H), 6.26 (d, J = 8.3 Hz, 1H), 4.34-4.10 (m, 3H), 3.55 (dt, J = 10.3, 8.4 Hz, 1H), 3.37 (dd, J = 17.7, 12.0 Hz, 1H), 3.26-3.08 (m, 1H), 2.76 (dd, J = 17.7, 5.1 Hz, 1H), 2.26-2.08 (m, 1H), 2.04 (s, 3H), 1.58 (s, 2H), 1.55-1.44 (m, 4H), 1.29 (t, J = 7.1 Hz, 3H), 0.90 (td, J = 7.4, 3.8 Hz, 6H).
13C NMR (75 MHz, CDCl3) δ 171.1, 166.4, 137.8, 129.5, 81.7, 77.6, 77.2, 76.7, 75.0, 60.8, 58.9, 49.3, 33.7, 26.2, 25.7, 23.6, 14.2, 9.6, 9.3. HR-ESI-MS calculated for C16H29O4N2 (M + H)+ 313.2122, found 313.2122.
References:
Albina?a, Carlos Berenguer;MacHara, Ale?;Rezacov, Pavlína;Pachl, Petr;Konvalinka, Jan;Kozísek, Milan [European Journal of Medicinal Chemistry,2016,vol. 121,p. 100 - 109]
367252-68-4
1 suppliers
inquiry

196618-13-0
213 suppliers
inquiry
312904-18-0
15 suppliers
inquiry

196618-13-0
213 suppliers
inquiry

204255-06-1
89 suppliers
inquiry

196618-13-0
213 suppliers
inquiry
651324-07-1
29 suppliers
inquiry

196618-13-0
213 suppliers
inquiry