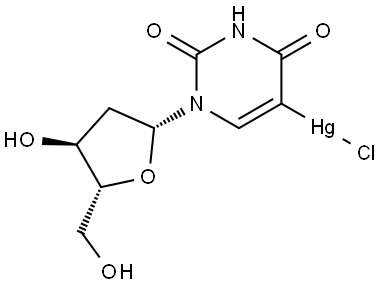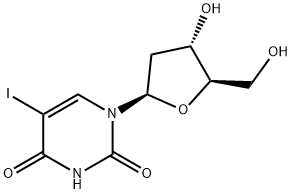
Idoxuridine synthesis
- Product Name:Idoxuridine
- CAS Number:54-42-2
- Molecular formula:C9H11IN2O5
- Molecular Weight:354.1
Idoxuridine is mainly used topically to treat herpes simplex keratitis.Idoxuridine can be synthesised by several related ways:
1) The reaction of 5-iodouracil (I) with refluxing POCl3 and dimethylaniline gives 2,4-dichloro-5-iodopyrimidine (II), which by reaction with NaOCH3 in refluxing methanol, is converted into 2,4-dimethoxy-5-iodopyrimidine (III). The reaction of (III) with 2-deoxy-3,6-di-Op-toluoyl-D-ribofuranosyl chloride (IV) in acetonitrile yields the ribofuranosyl pyridone (V ), which is demethylated with acetic anhydride – dry HCl to afford 2′-deoxy-5-iodo-3‘, 6′-di-Op-toluoyluridine (VI) Finally, this compound is hydrolyzed with NaOCH3 in methanol.
3) The iodination of 2′-deoxyuridine (IX) with iodine, iodic acid, acetic anhydride, CCl4 and water gives 2′-deoxy-5,6-diiodo-5,6-dihydrouridine (X), which is then treated with NaOH to eliminate HI.
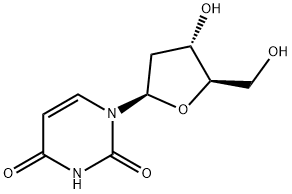
951-78-0
494 suppliers
$5.00/100mg

696-07-1
300 suppliers
$17.00/5g

54-42-2
453 suppliers
$6.00/250mg
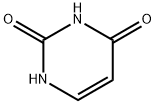
66-22-8
802 suppliers
$5.00/10g
Yield:-
Reaction Conditions:
with thymidine phosphorylase from Escherichia coli (E.C. 2.4.2.4) immobilized on Sepabeads EC-EP in aq. phosphate buffer; pH=7.5 at 20; for 3 h;Enzymatic reaction;Reagent/catalyst;
Steps:
2.7. Transglycosylation: general protocol
General procedure: To a solution of phosphate buffer (10 mM) at pH 7.5 (10 mL) containing 2′-deoxyuridine (1, 45.6 mg, 20 mM) and the 5-substituted base (8-13, 13-22 mg, 10 mM), 10 IU of immobilized enzyme (EcTP or BsPyNP) were added and the suspension was kept under mechanical stirring at room temperature. In the case of 5-bromovinyluracil (13), pH was set to 10 by using a 10 mM phosphate buffer containing 13.8 mg of potassium carbonate (final concentration 10 mM). Aliquots (0.2 mL) were periodically withdrawn, filtered through a pipette filter device to remove the biocatalyst and analyzed by HPLC (see below for chromatographic conditions and Rt). When the highest conversion was achieved, the reaction was stopped by filtration of the immobilized biocatalyst on a Büchner funnel with a sintered glass disc under reduced pressure. The produced nucleosides (2 and 14-18) were identified by comparison of their HPLC Rt with that of authentic samples.
References:
Serra, Immacolata;Bavaro, Teodora;Cecchini, Davide A.;Daly, Simona;Albertini, Alessandra M.;Terreni, Marco;Ubiali, Daniela [Journal of Molecular Catalysis B: Enzymatic,2013,vol. 95,p. 16 - 22]

951-78-0
494 suppliers
$5.00/100mg

54-42-2
453 suppliers
$6.00/250mg
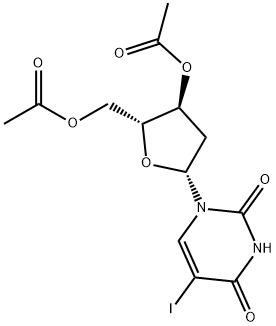
1956-30-5
37 suppliers
$130.00/100mg

54-42-2
453 suppliers
$6.00/250mg

696-07-1
300 suppliers
$17.00/5g

50-89-5
634 suppliers
$9.00/1g

54-42-2
453 suppliers
$6.00/250mg
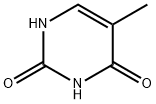
65-71-4
515 suppliers
$5.00/5g