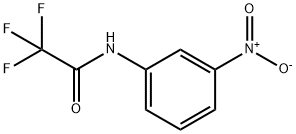
Iodine synthesis
- Product Name:Iodine
- CAS Number:7553-56-2
- Molecular formula:I2
- Molecular Weight:253.81
Yield:7553-56-2 66%
Reaction Conditions:
Stage #1: N-trifluoroacetic-3-nitroanilinewith sodium hydride in tetrahydrofuran at 0; for 1 h;
Stage #2: methyl iodide in tetrahydrofuran at 20;
Steps:
9
EXAMPLE 9 Synthesis of Compound 97 A solution of nitroaniline (1.0 g, 7.13 mmole) in CH2Cl2 (25 ML) was treated with pyridine (2.9 ML, 36 mmole) and trifluoroacetic anhydride (5 ML, 36 mmole) and stirred at ambient temperature for 3 hours.. The reaction was diluted further with CH2Cl2, washed with IN HCl and brine, dried (MgSO4) and concentrated in vacuo to yield I1 (1.61 g, 95%) as a white solid.. The 1H NMR was consistent with that of the desired structure. . To a slurry of NaH (60% oil dispersion; 34 mg, 1.42 mmole) in THF (10 ML) at 0° C. was added a solution of I1 (200 mg, 0.85 mmole) in THF (10 ML) and the mixture stirred for 1 hour.. To this was added methyl iodide (100 μL, 1.7 mmole) and the mixture was stirred overnight at ambient temperature.. The reaction was poured into water and extracted with EtOAc. The organics were separated, dried (MgSO4) and concentrated in vacuo.. Pure I2 was obtained through flash chromatography, eluding with 5% EtOAc in hexanes, in a yield of 163 mg (66%) as a yellow solid..
References:
US6344465,2002,B1 Location in patent:Page column 30
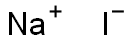
7681-82-5
634 suppliers
$10.00/25g

7553-56-2
626 suppliers
$19.00/25g
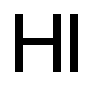
10034-85-2
340 suppliers
$40.89/25g

7553-56-2
626 suppliers
$19.00/25g
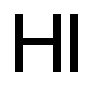
14362-44-8
2 suppliers
inquiry

7553-56-2
626 suppliers
$19.00/25g
10034-85-2
340 suppliers
$40.89/25g

1333-74-0
115 suppliers
$190.00/56L

7553-56-2
626 suppliers
$19.00/25g