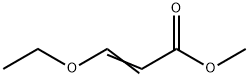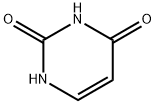
Uracil synthesis
- Product Name:Uracil
- CAS Number:66-22-8
- Molecular formula:C4H4N2O2
- Molecular Weight:112.09
Yield:66-22-8 95.2%
Reaction Conditions:
Stage #1: ureawith sodium methylate in 5,5-dimethyl-1,3-cyclohexadiene at 60; for 4 h;
Stage #2: methyl 3-methoxyprop-2-enoate in 5,5-dimethyl-1,3-cyclohexadiene at 80 - 110; for 6 h;Temperature;Time;Solvent;Reagent/catalyst;
Steps:
1-5; 6-13 Example 5
General procedure: 12.0 g (0.2 mol) of urea and 10.8 g (0.2 mol) of sodium methoxide were added to different amounts of xylene, and the mixture was stirred and heated to 60° C. to react for 4 hours. After the reaction, the xylene and methanol are distilled out, and dried under vacuum to obtain sodium urea salt. In a 500ml three-necked flask, add 9.02g (0.11mol) of urea sodium salt, (0.10mol) of compound formula II, 120g of xylene, and add 0.05g of polyoxyethylene lauryl ether phosphate sodium salt, The temperature is raised to 80°C and the reaction is carried out for 4 hours under stirring, the temperature is increased to 110°C, and the reaction is carried out for 2 hours, and the alcohol or ammonia substances are distilled out. After the reaction is over, add 100ml of water after cooling to 50°C, dissolve it and separate the liquids, recover the upper layer of xylene, add hydrochloric acid to acidify the lower layer of water, and let stand for 4 hours at room temperature. A large amount of crystals are precipitated out, filtered, and the filter cake is washed three times with cold water. A total of 50ml of water is dried to obtain uracil. The purity of uracil was 99.1% by HPLC, and the yield of uracil was calculated to get the following table.
References:
CN112645886,2021,A Location in patent:Paragraph 0046-0048; 0049-0052; 0053-0055; 0056-0086
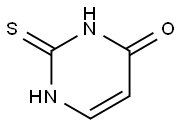
141-90-2
482 suppliers
$6.00/1g

66-22-8
805 suppliers
$5.00/10g
201230-82-2
1 suppliers
inquiry

696-07-1
300 suppliers
$18.00/5g
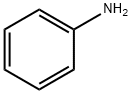
62-53-3
664 suppliers
$10.00/1g

66-22-8
805 suppliers
$5.00/10g
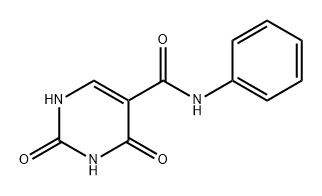
65906-66-3
0 suppliers
inquiry