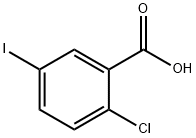
2-Chloro-5-iodobenzoic acid synthesis
- Product Name:2-Chloro-5-iodobenzoic acid
- CAS Number:19094-56-5
- Molecular formula:C7H4ClIO2
- Molecular Weight:282.46

118-91-2
648 suppliers
$14.00/25g
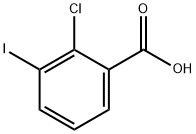
874817-93-3
47 suppliers
$31.00/100mg

19094-56-5
520 suppliers
$6.00/5g
Yield:19094-56-5 394 kg
Reaction Conditions:
with ammonium peroxodisulphate;sulfuric acid;iodine;glacial acetic acid at 60 - 85;Large scale;Reagent/catalyst;Temperature;
Steps:
1-3; 5-6; 8-10 Example- 10
In a clean reactor of 3000 lit capacity equipped with stirrer, thermometer, condenser and addition funnel charge, 2-chlorobenzoic acid (123.2 kg), acetic acid (460 lit), and iodine (100kg) and ammonium persulfate (140kg) were added. The reaction mass was stirred at room temperature for 30minutes so as to make a uniform slurry. Concentrated sulfuric acid (150 Kg)was charged in an addition tank and dripped into the reactor at a constant rate so as to maintain the temperature of the reaction mass below 60°C. The temperature was slowly raised to 80°C to 85°C and maintained for three hours. Samples were withdrawn every hour and analyzed and recorded. At the end of third hour the starting material was found to be less than 1%. The colour of iodine in the solution slowly disappeared at this stage, mass becomes pale yellow and a pale yellow solid started to precipitate. (0101) Now the reaction mass is cooled to 60°C and water (1200 lit) was added to it slowly so as to maintain the temperature around 60°C. Once the water was added, 5% sodium thiosulfate solution (lOOlit) was added to completely remove the iodine. If required more thiosulfate solution may be added. The stirring was slowed down and the mass was cooled to 15-20°C. The precipitated solid was filtered off, washed with copious amount of water to remove the acetic acid. The product was removed from the filter and dried in vacuum at 55°C for 6 hours. The HPTC analysis of the crude product sample had the following composition: 2-chlorobenzoic acid (0.82%), 2-chloro-5-iodobenzoic acid(96.25%), 2-chloro-3-iodobenzoic acid (2.3%) and 2-chloro-3,5-diiodobenzoic acid (0.11%)%. (0102) The product was purified from toluene. The yield of the product was 124.4 kg (1.24w/w based on iodine input). The following reaction analysis is obtained after purification as provided in Table 9. (0103) TABLE 9 (0104) Example- 10 In a clean reactor of 3000 litre capacity equipped with stirrer, thermometer, condenser and addition funnel charge, 2-chlorobenzoic acid (378 kg), acetic acid (1260 lit), and iodine (300kg) and ammonium persulfate (420kg) were added. The reaction mass was stirred at room temperature for 30minutes so as to make a uniform slurry. Concentrated sulfuric acid (690Kg)was charged in an addition tank and dripped into the reactor at a constant rate so as to maintain the temperature of the reaction mass below 60°C. The temperature was slowly raised to 80°C to 85°C and maintained for 3-4 hours. At the end of third hour the starting material was found to be less than 1%. The colour of iodine in the solution slowly disappeared at this stage, mass become pale yellow and a pale yellow solid started to precipitate. (0105) Now the reaction mass was cooled to 60°C and water (1800 lit) was added to it slowly so as to maintain the temperature around 60°C. Once the water was added, 5% sodium thiosulfate solution (3001it) was added to completely remove the iodine. The product is filtered to obtain the crude material. The yield of the product obtained is 840.0kg(wet). (0106) The HPTC analysis of the crude product sample had the following composition: 2-chlorobenzoic acid -0.34%, 2-chloro-5-iodobenzoic acid -84.21%, 2-chloro-3- iodobenzoic acid -13.51% and 2-chloro-3,5-diiodobenzoic acid - (0107) To the crude product thus obtained was subjected to first round of purification, thus acetic acid (690.01it) and water (3001it) was added to the crude product and heated to 80°C for dissolution into a clear solution. The clear solution was cooled to 10°C to obtain 2-chloro5-iodobenzoic acid of purity greater than 98% by HPTC. (0108) The purified product was further subjected to a second round of purification using toluene. The product was taken in toluene (8001it) and heated to reflux so as to dissolve most of the iodinated product formed. The aqueous and organic layers were separated at this stage and the organic layer was washed with water and a 1% solution of sodium bi carbonate to remove any acidic material and finally with water. The toluene layer was treated with charcoal and filtered hot. The toluene layer was slowly cooled to room temperature and then to 10°C and kept at that temperature for 1 hour. The precipitated mass was filtered off to obtain 2-chloro-5-iodobenzoic acid of formal II with high purity levels. (0109) The highly purified product of formula II after second round of purification with toluene hadan yield of 394.00 kg, having a melting point of 158-161°C. Analysis of the highly purified product of formula II had the following composition as provided in Table 10.
References:
WO2022/74631,2022,A1 Location in patent:Page/Page column 21-26; 28-32

118-91-2
648 suppliers
$14.00/25g

19094-56-5
520 suppliers
$6.00/5g

89-54-3
248 suppliers
$13.00/5g

19094-56-5
520 suppliers
$6.00/5g
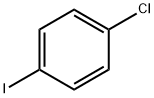
637-87-6
289 suppliers
$38.00/10g
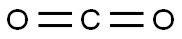
124-38-9
127 suppliers
$214.00/14L

19094-56-5
520 suppliers
$6.00/5g
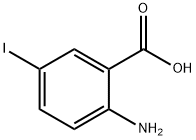
5326-47-6
323 suppliers
$8.00/5g

19094-56-5
520 suppliers
$6.00/5g