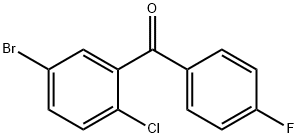
(5-bromo-2-chlorophenyl)(4-fluorophenyl)methanone synthesis
- Product Name:(5-bromo-2-chlorophenyl)(4-fluorophenyl)methanone
- CAS Number:915095-85-1
- Molecular formula:C13H7BrClFO
- Molecular Weight:313.55

462-06-6
419 suppliers
$10.00/5g
21739-92-4
655 suppliers
$8.00/10g

915095-85-1
160 suppliers
inquiry
Yield:915095-85-1 96.94%
Reaction Conditions:
Stage #1: fluorobenzene;5-bromo-2-chlorobenzoic acidwith oxalyl dichloride;N,N-dimethyl-formamide at 0 - 25;
Stage #2: with Aluminum Chloride at 25;
Steps:
Characterization and synthesis of compounds 1, 3, and 4.
With regard to a slurry 2 (0.0354 mol, 8.34 g), toa catalytic amount of DMF (0.7 mmol, 0.055 mL) along with fluorobenzene (50 mL) oxalyl chloride (0.0372 mol, 3.24 mL)was added dropwise. Then we stirred it for two hours at 21-25 °C, and concentrated to a low volume (30 mL). An additionalamount of fluorobenzene (20 mL) was added. Then we cooled the solution to 0-5 °C. We added AlCl3 to this solution in threeportions (5.19 g, 0.0389 mol), keeping the internal temperature <25 °C. We gathered the solids through filtration, and thendried them in an oven to obtain compound 3 as white crystals (10.77 g, 96.94%). 1H NMR under 400 MHz (CDCl3) δ:7.19-7.13 (m, 2H), 7.34 (d, 1H), 7.50 (d, 1H), 7.57 (dd, 1H),7.83-7.81 (m, 2H).1 M potassium tert-butoxide (0.0370 mol, 3.7 mL) was added dropwise to the mixture of compound 3 (0.336 mol,10.54 g) with (S)-tetrahydrofuran-3-ol (0.0336 mol, 2.96 g) in THF (36 mL). Then we stirred this mixture for 30 min at7-10 °C, and quenched it with water (35 mL). MTBE (35 mL) was added to the residue. Then all the layers were parted. Thewhole organic was vacuum concentrated. IPA (25 mL) and water (2.3 mL) were added to the residue at 60 °C for 60 min, andthe slurry was gradually cooled to 0-5 °C. We gathered the solids through filtration and then dried them in an oven to obtaincompound 4 as white crystals (10.93 g, 85.19%). 1H NMR under 400 MHz (CDCl3) δ: 2.19-2.12 (m, 1H), 2.31-2.20 (m, 1H),3.94-3.89 (m, 1H), 4.05-3.97 (m, 3H), 5.01 (m, 1H), 6.91 (d, 2H), 7.32 (d, 1H), 7.48 (d, 1H), 7.54 (dd, 1H),7.76 (d, 2H).The solution of compound 4 (0.0231 mol, 8.81 g) with AlCl3 (0.0463 mol, 6.17 g) in toluene (45 mL) wassupplemented with 1,1,3,3-tetramethyldisiloxane (4.04 g, 0.0301 mol) at <20 °C. We stirred this mixture for 1.5 h at 20-23 °C. Then we cooled this mixture to 0-5 °C, supplemented it with ice water (40 mL) for 15 min, and separated the layers.The residue was supplemented with acetonitrile (40 mL) and water (20 mL). We cooled the resulting mixture to 0-3 °C andstirred for 2 h. The solids were collected by filtration and dried in an oven to give 1 as white crystals (7.75 g, 91.28%).1H NMR under 400 MHz (CDCl3) δ: 2.19-2.12 (m, 1H), 2.31-2.20 (m, 1H), 3.94-3.89 (m, 1H), 4.05-3.97 (m, 3H), 5.01 (m,1H), 6.91 (d, 2H), 7.32 (d, 1H), 7.48 (d, 1H), 7.54 (dd, 1H),7.76 (d, 2H). HRMS (ESI+), m/z: calcd for C17H16BrClO2388.9920 [M+Na+]; found 388.9939.
References:
Li, H.;Li, X. X.;Shen, L.;Shi, E. H.;Wang, L. R.;Zhang, C. Y.;Zhang, D. L.;Zhao, S. [Journal of Structural Chemistry,2020,vol. 61,# 7,p. 1167 - 1174][Zh. Strukt. Kim.,2020,vol. 61,# 7,p. 1230 - 1236,7]

462-06-6
419 suppliers
$10.00/5g

21900-52-7
82 suppliers
$12.00/250mg

915095-85-1
160 suppliers
inquiry

21739-92-4
655 suppliers
$8.00/10g

915095-85-1
160 suppliers
inquiry

104-92-7
429 suppliers
$10.00/10g

915095-85-1
160 suppliers
inquiry

403-43-0
464 suppliers
$9.00/25g

915095-85-1
160 suppliers
inquiry