
(2-Chloro-5-iodophenyl)[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methanone synthesis
- Product Name:(2-Chloro-5-iodophenyl)[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methanone
- CAS Number:915095-87-3
- Molecular formula:C17H14ClIO3
- Molecular Weight:428.65

To a solution of the fluoride VIII.1 (208kg), tetrahydrofuran (407kg) and (S)-3- hydroxytetrahydrofuran (56kg) is added potassium-terf-butanolate solution (20%) in tetrahydrofuran (388kg) within 3 hrs at 16 to 25°C temperature. After completion of the addition, the mixture is stirred for 60min at 20°C temperature. Then the conversion is determined via HPLC analysis. Water (355kg) is added within 20 min at a temperature of 21 °C (aqueous quench). The reaction mixture is stirred for 30 min (temperature: 20°C). The stirrer is switched off and the mixture is left stand for 60 min (temperature: 20°C). The phases are separated and solvent is distilled off from the organic phase at 19 to 45°C temperature under reduced pressure. 2- Propanol (703kg) is added to the residue at 40 to 46°C temperature and solvent is distilled off at 41 to 50°C temperature under reduced pressure. 2-Propanol (162kg) is added to the residue at 47°C temperature and solvent is distilled off at 40 to 47°C temperature under reduced pressure. Then the mixture is cooled to 0°C within 1 hr 55 min. The product is collected on a centrifuge, washed with a mixture of 2- propanol (158kg) and subsequently with terf.-butylmethylether (88kg) and dried at 19 to 43°C under reduced pressure. 227kg (91 ,8%) of product are obtained as colourless solid. The identity of the product is determined via infrared spectrometry.
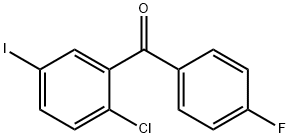
915095-86-2
217 suppliers
$5.00/250mg
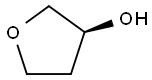
86087-23-2
648 suppliers
$5.00/1g
![(2-Chloro-5-iodophenyl)[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methanone](/CAS/20150408/GIF/915095-87-3.gif)
915095-87-3
174 suppliers
inquiry
Yield:915095-87-3 91.8%
Reaction Conditions:
with potassium tert-butylate in tetrahydrofuran at 16 - 25; for 3 h;
Steps:
2
Example 2: Synthesis of the ketone VII.1To a solution of the fluoride VIII.1 (208kg), tetrahydrofuran (407kg) and (S)-3- hydroxytetrahydrofuran (56kg) is added potassium-terf-butanolate solution (20%) in tetrahydrofuran (388kg) within 3 hrs at 16 to 25°C temperature. After completion of the addition, the mixture is stirred for 60min at 20°C temperature. Then the conversion is determined via HPLC analysis. Water (355kg) is added within 20 min at a temperature of 21 °C (aqueous quench). The reaction mixture is stirred for 30 min (temperature: 20°C). The stirrer is switched off and the mixture is left stand for 60 min (temperature: 20°C). The phases are separated and solvent is distilled off from the organic phase at 19 to 45°C temperature under reduced pressure. 2- Propanol (703kg) is added to the residue at 40 to 46°C temperature and solvent is distilled off at 41 to 50°C temperature under reduced pressure. 2-Propanol (162kg) is added to the residue at 47°C temperature and solvent is distilled off at 40 to 47°C temperature under reduced pressure. Then the mixture is cooled to 0°C within 1 hr 55 min. The product is collected on a centrifuge, washed with a mixture of 2- propanol (158kg) and subsequently with terf.-butylmethylether (88kg) and dried at 19 to 43°C under reduced pressure. 227kg (91 ,8%) of product are obtained as colourless solid. The identity of the product is determined via infrared spectrometry.
References:
WO2011/39107,2011,A1 Location in patent:Page/Page column 19-20

1459754-40-5
17 suppliers
inquiry
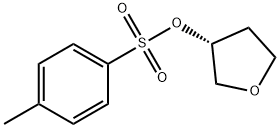
219823-47-9
115 suppliers
inquiry
![(2-Chloro-5-iodophenyl)[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methanone](/CAS/20150408/GIF/915095-87-3.gif)
915095-87-3
174 suppliers
inquiry
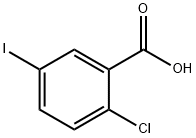
19094-56-5
520 suppliers
$6.00/5g
![(2-Chloro-5-iodophenyl)[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methanone](/CAS/20150408/GIF/915095-87-3.gif)
915095-87-3
174 suppliers
inquiry

462-06-6
414 suppliers
$10.00/5g
![(2-Chloro-5-iodophenyl)[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methanone](/CAS/20150408/GIF/915095-87-3.gif)
915095-87-3
174 suppliers
inquiry

281652-58-2
21 suppliers
inquiry
![(2-Chloro-5-iodophenyl)[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methanone](/CAS/20150408/GIF/915095-87-3.gif)
915095-87-3
174 suppliers
inquiry