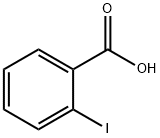
2-Iodobenzoic acid synthesis
- Product Name:2-Iodobenzoic acid
- CAS Number:88-67-5
- Molecular formula:C7H5IO2
- Molecular Weight:248.02
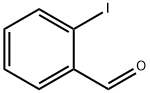
26260-02-6
242 suppliers
$8.00/250mg

88-67-5
542 suppliers
$5.00/5g
Yield:88-67-5 77%
Reaction Conditions:
with [2,2]bipyridinyl;lithium hydroxide monohydrate;oxygen;sodium hydroxide at 50; under 760.051 Torr; for 10 h;Sealed tube;
Steps:
3.4. General Procedure for the Oxidation of Aldehyde 1
General procedure: Aldehyde 1 (0.2 mmol), Ag/C3N4 (10 mol%), NaOH (1 equiv), H2O (1 mL) were added into a 10 mL sealed tube under O2 (1 atm). The mixture was stirred at 50o C for 10 h. Then the reaction was stopped and 1M dilute hydrochloric acid was added to adjust the pH value to 3. The mixture was extracted with ethyl acetate (3 × 3 mL). The combined organic phase was dried with Na2SO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography (5:1 hexanes and ethyl acetate).
References:
Wu, Chaolong;Yao, Xiaoquan;Yu, Min;Zhou, Li;Zhu, Li [Letters in Organic Chemistry,2021,vol. 18,# 3,p. 167 - 175]
118-92-3
4 suppliers
$23.00/250g

88-67-5
542 suppliers
$5.00/5g
4840-91-9
22 suppliers
$145.00/250mg

88-67-5
542 suppliers
$5.00/5g
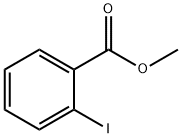
610-97-9
414 suppliers
$9.00/1g

88-67-5
542 suppliers
$5.00/5g
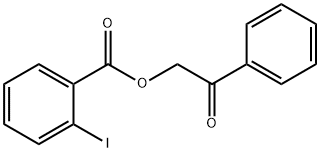
55153-31-6
3 suppliers
inquiry

88-67-5
542 suppliers
$5.00/5g