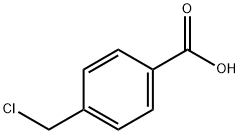
4-(Chloromethyl)benzoic acid synthesis
- Product Name:4-(Chloromethyl)benzoic acid
- CAS Number:1642-81-5
- Molecular formula:C8H7ClO2
- Molecular Weight:170.59
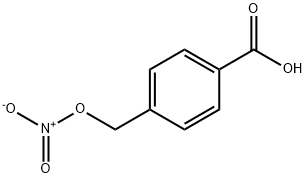
258278-55-6
0 suppliers
inquiry

1642-81-5
386 suppliers
$17.00/5g
Yield:1642-81-5 90.2%
Reaction Conditions:
with silver nitrate;sulfuric acid in acetonitrile for 7.25 h;Inert atmosphere;Industry scale;Darkness;Reflux;
Steps:
1
Example 1: Synthesis of 4-nitro-oxy-methyl-benzoic acid (I); a) Reaction of 4-chloromethyl-benzoic acid (III) with AqNO and in the presence of H?SC>49.29 kg of 4-chloromethyl-benzoic acid (III) were added to 92.9 I of acetonitrile with stirring for 20 minutes, under a slow nitrogen current. 93 ml of sulphuric acid were added and the mixture was stirred for 15 minutes. 13.65 kg of silver nitrate were added, following the same operation conditions as when adding (III). The reactor was protected from direct exposure to light and the mixture was stirred for 15 minutes.The mixture was then refluxed for 7 hours and 15 minutes. The reaction mix was cooled down to 20°C-25°C. 37.2 I of dimethylformamide were added and it was stirred for 30 minutes, keeping the temperature between 25°C and 20°C.b) Separation of the silver salts by filtrationThe silver salts were separated by filtration, under nitrogen pressure, through a filter containing 9 kg of cellulose, previously washed with 1 1 1 I of water and three times with 28 I of dimethylformamide. The separated solid waste was washed twice with 9.3 I of dimethylformamide. The cellulose was withdrawn from the filter and washed with dimethylformamide until running clear and it was then rinsed with water,c) Precipitation with water The liquid phases were put together and the temperature was stabilised to between 25°C and 20°C. 1486 I of water were added for 1 hour, maintaining the temperature between 20°C and 25°C. The mixture was stirred for 1 hour, maintaining the temperature between 20°C and 25°C. The precipitate was separated by filtration, and the cake thus obtained was washed with water until obtaining a pH similar to that of the water. The cake was finally washed with 18.6 I of ethanol.ngThe wet solid was dried at a temperature of not more than 40°C in a vacuum until the KF water content was of 0.2% at the most. 9.68 kg of4-nitro-oxy-methyl-benzoic acid (I) were obtained. Yield 90.2%. HPLC Purity 99.35%. Content of (IV) 0.23%.
References:
FERRER INTERNACIONAL, S.A.;NICOX S. A.;ANGLADA, Luis;PALOMER, Albert;SOBRAL, Luis WO2011/58162, 2011, A1 Location in patent:Page/Page column 7-8
99-94-5
591 suppliers
$9.00/1g

1642-81-5
386 suppliers
$17.00/5g
3006-96-0
290 suppliers
$14.00/1g

1642-81-5
386 suppliers
$17.00/5g
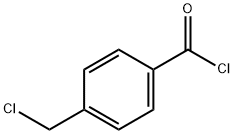
876-08-4
270 suppliers
$11.00/25g

1642-81-5
386 suppliers
$17.00/5g
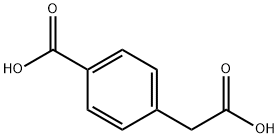
501-89-3
139 suppliers
$7.00/1g

1642-81-5
386 suppliers
$17.00/5g