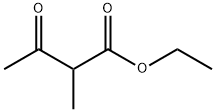
| Identification | More | [Name]
Ethyl 2-methylacetoacetate | [CAS]
609-14-3 | [Synonyms]
2-ACETYLPROPIONIC ACID ETHYL ESTER
2-METHYLACETOACETIC ACID ETHYL ESTER
ETHYL 2-ACETYLPROPIONATE
ETHYL 2-METHYLACETOACETATE
2-methyl-3-oxo-butanoicaciethylester
Acetoacetic acid, 2-methyl-, ethyl ester
alpha-Methylacetoacetic ester
Ethyl 2-methyl-3-oxobutanoate
Ethyl 2-methyl-3-oxobutyrate
Ethyl alpha-acetylpropionate
Ethyl alpha-methylacetoacetate
Ethyl alpha-methylacetylacetate
Ethyl ester of 2-methyl-3-oxobutanoic acid
Ethyl methylacetoacetate
Alpha-(Aminomethyl)-4-Hydroxy-1,3-Benzenedimethanol
Ethyl 2-methylacetacetate
ETHYL 2-METHYL-AETACETATE
(R,S)-2-Methyl-3-oxo-butyric acid ethyl ester
2-methyl-3-oxo-butanoic acid ethyl ester
Butanoic acid, 2-methyl-3-oxo-, ethyl ester | [EINECS(EC#)]
210-179-9 | [Molecular Formula]
C7H12O3 | [MDL Number]
MFCD00009164 | [Molecular Weight]
144.17 | [MOL File]
609-14-3.mol |
| Safety Data | Back Directory | [Safety Statements ]
S24/25:Avoid contact with skin and eyes . | [RIDADR ]
1993 | [WGK Germany ]
3
| [TSCA ]
Yes | [HS Code ]
29182300 |
| Hazard Information | Back Directory | [Chemical Properties]
clear colorless to yellow liquid | [Uses]
Ethyl 2-methylacetoacetate is used in Japp-Klingemann reaction to prepare hydrazones from beta-keto acid and aryl diazonium salts. It is used as a precursor involved in the synthesis of ethylpyruvate phenylhydrazone via reaction with benzenediazonium chloride. |
|
|