sacubitril synthesis
- Product Name:sacubitril
- CAS Number:1038924-61-6
- Molecular formula:C17H17NO
- Molecular Weight:251.32
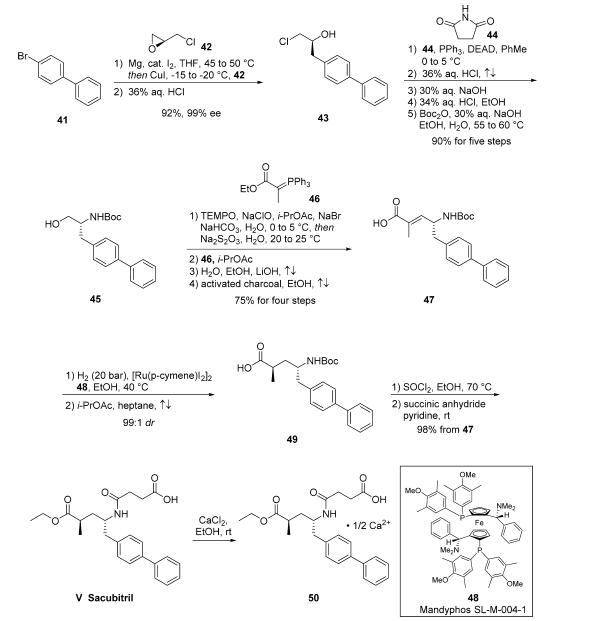
Subsequently, activation of the acid as the acid halide through the use of thionyl chloride and ethanol not only reestablished the ethyl ester but removed the Boc group, revealing a primary amine which then reacted with succinic anhydride to ultimately deliver sacubitril (V). The freebase form of sacubitril does not readily crystallize; the isolation of a number of pharmaceutically acceptable salts of sacubitril via crystallization, most preferably the calcium salt 50 or sodium salts, have been reported.
Preparation of the sacubitril/valsartan supramolecular complex (trisodium salt, hemihydrate) has been described on a kilo-scale from sacubitril calcium salt via neutralization to the freebase and subsequent complexation with valsartan in iPrOAc/ acetone. Addition of NaOH and crystallization then provided the desired trisodium salt hemihydrate.
72479-05-1
108 suppliers
$18.00/250mg
144432-80-4
129 suppliers
$12.00/1g

1038924-61-6
63 suppliers
$435.00/1g
Yield: 95.9%
Reaction Conditions:
with potassium carbonate in water;dimethyl sulfoxide at 80;Temperature;
Steps:
1
Compound III (3.28 g, 20 mmol, X = Br) was dissolved in a mixture of DMSO (50 ml) and water (10 ml)Compound II (7.87 g, 28.1 mmol) and potassium carbonate (11 g, 80 mmol) were sequentially added.After the reaction solution was degassed, the mixture was heated to 80 ° C and stirred overnight (12 to 20 hours).TLC point plate, the raw material completely reacted.The reaction mixture was filtered through celite and the residue was washed with ethyl acetate (500 ml).The filtrate was washed with 10% ammonium chloride solution, saturated brine,The organic phase was dried over anhydrous sodium sulfate, filtered with suction,The filtrate was concentrated under reduced pressure to give 4.82 g of a white solid in a yield of 95.9%.
References:
Jiangsu Furui Bio-pharmaceutical Co., Ltd.;Chen Benshun CN104926707, 2017, B Location in patent:Paragraph 0036; 0038; 0060-0061
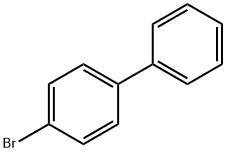
92-66-0
564 suppliers
$10.00/1g

72479-05-1
108 suppliers
$18.00/250mg

1038924-61-6
63 suppliers
$435.00/1g
![(S)-5-[(Biphenyl-4-yl)carbonyl]pyrrolidin-2-one](/CAS/20150408/GIF/195137-95-2.gif)
195137-95-2
24 suppliers
inquiry

1038924-61-6
63 suppliers
$435.00/1g
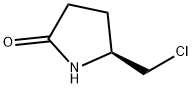
72479-04-0
8 suppliers
inquiry

144432-80-4
129 suppliers
$12.00/1g

1038924-61-6
63 suppliers
$435.00/1g
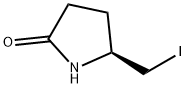
29266-73-7
2 suppliers
inquiry
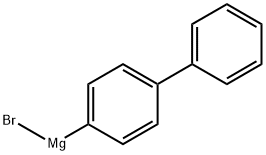
3315-91-1
67 suppliers
$153.00/50mL

1038924-61-6
63 suppliers
$435.00/1g