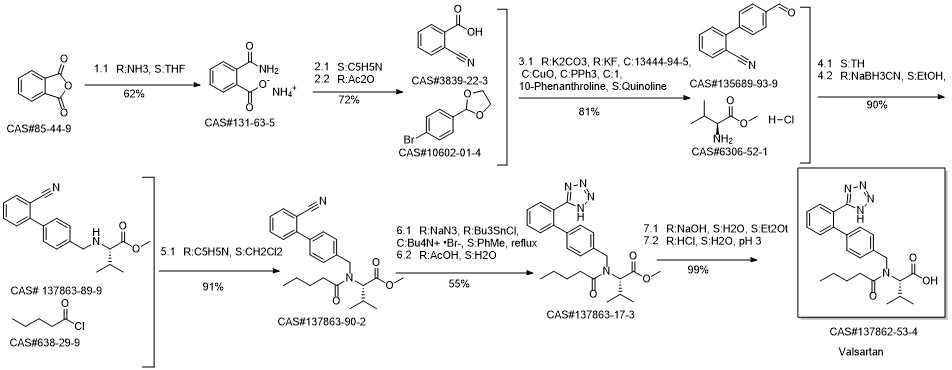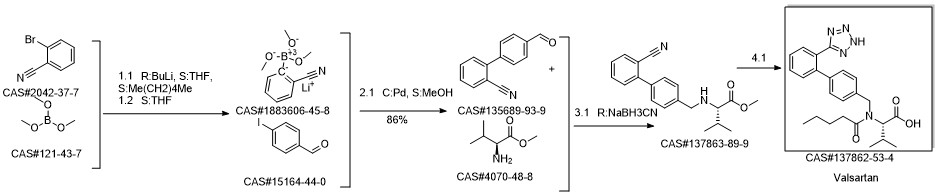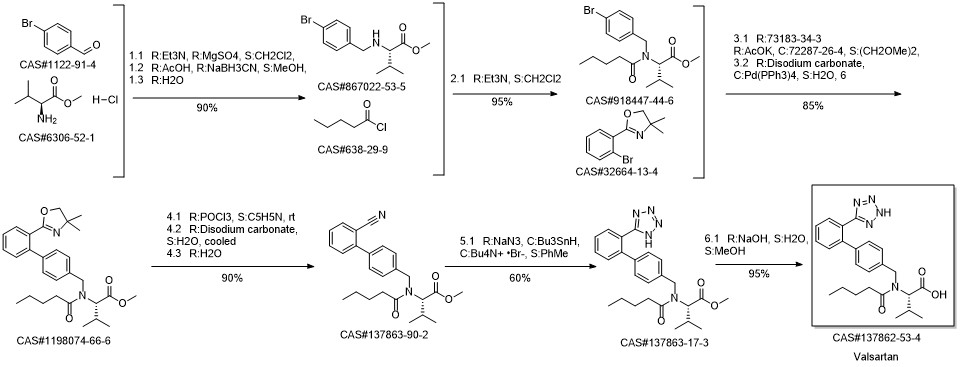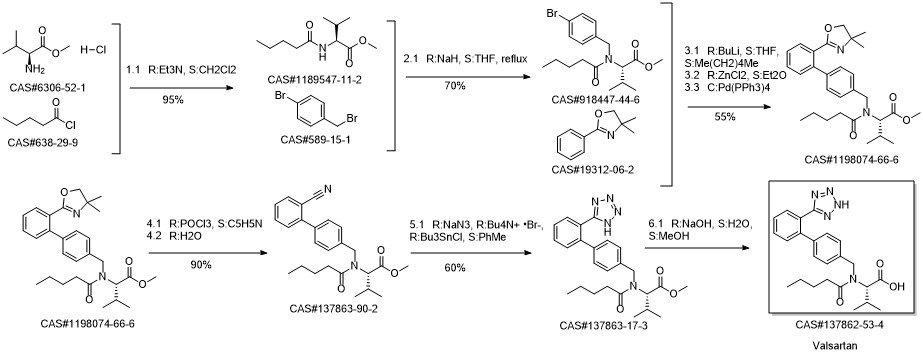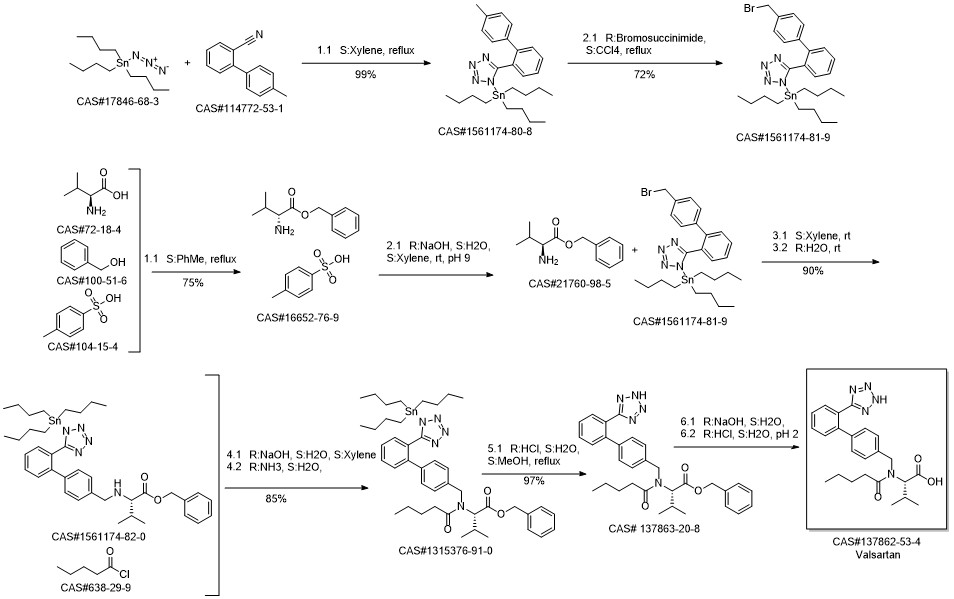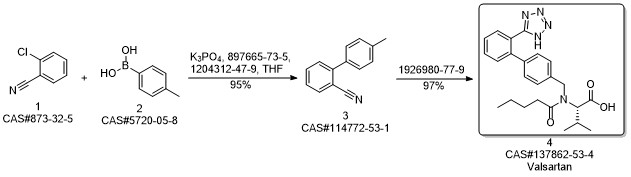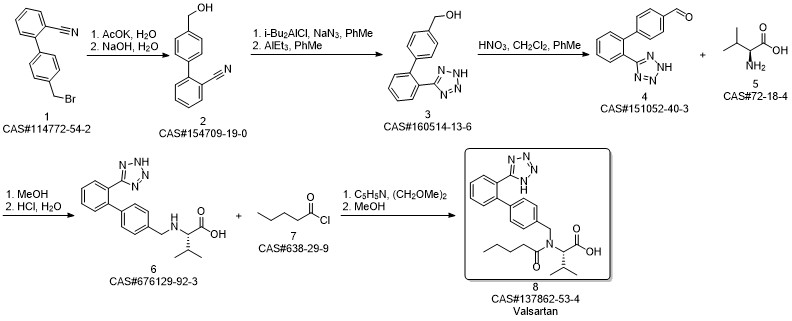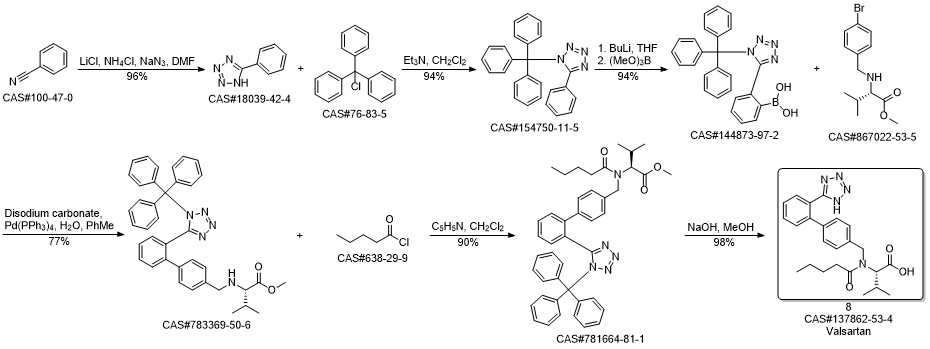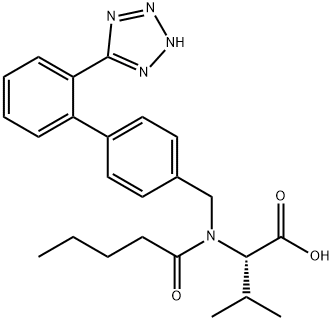
Valsartan synthesis
- Product Name:Valsartan
- CAS Number:137862-53-4
- Molecular formula:C24H29N5O3
- Molecular Weight:435.52
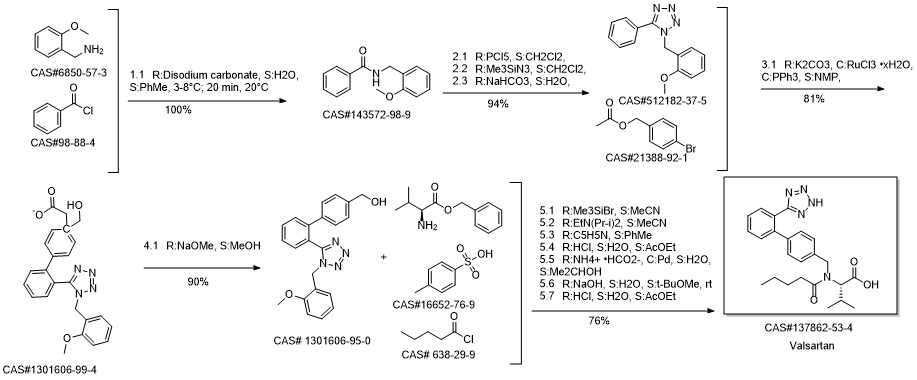
Reference: Seki, Masahiko; Nagahama, Masaki. Synthesis of Angiotensin II Receptor Blockers by Means of a Catalytic System for C-H Activation. Journal of Organic Chemistry. Volume 76. Issue 24. Pages 10198-10206. Journal; Online Computer File. (2011)
![(S)-Methyl 3-methyl-2-(N-((2'-(1-trityl-1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)pentanamido](/CAS/GIF/781664-81-1.gif)
781664-81-1
13 suppliers
$466.51/1g

137862-53-4
905 suppliers
$9.00/10g
Yield:137862-53-4 95%
Reaction Conditions:
Stage #1: N-[[2'-(1-triphenylmethyltetrazol-5yl)biphenyl-4-yl]methyl]-N-valeryl-L-valine methyl esterwith methanol;potassium hydroxide for 4 h;Heating / reflux;
Stage #2: with water for 5 h;Heating / reflux;
Steps:
3 Preparation of crude valsartan (I)
1.2 g of methyl (S)-3-methyl-2-(pentanoyl-(2'-(1-triphenylmethyl-tetrazol-5-yl)-biphenyl-4-ylmethyl)amino) butanoate, prepared as in example 2, are dissolved in 10.7 ml of methanol and 0.16 g (2.9 mmol) of pulverized KOH are added. The mixture is first heated under reflux for 4 h, and then 2.5 ml 2M KOH are added. The mixture is then heated for another 5h under reflux. After the completion of the reaction the evaporable components are distilled off and the water phase is acidified to pH 1 and extracted with tert-butyl methyl ether (2 * 12 ml). The organic phase is washed twice with 16 ml of water, dried over Na2SO4 and evaporated to leave a dry residue. 0.72 g of crude valsartan are isolated (HPLC Area %: 98.0 %, yield: 95 %, yield based on compound (II): 92 %). By contrast, the yield in the deprotection step (Example 3 of WO 2004/094391) is only 81%.
References:
EP1661891,2006,A1 Location in patent:Page/Page column 13
![N-[(2′-(1-Triphenylmethyl-Tetrazole-5-Yl)Biphenyl-4-Yl]-Methyl]-N-Valeryl-L- Valine Benzyl Ester](/CAS/20200611/GIF/137864-44-9.gif)
137864-44-9
3 suppliers
inquiry

137862-53-4
905 suppliers
$9.00/10g
![5-Oxazolidinone, 4-(1-methylethyl)-3-(1-oxopentyl)-2-[2'-[2-(triphenylmethyl)-2H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]-, (4S)-](/CAS/20210305/GIF/852212-82-9.gif)
852212-82-9
0 suppliers
inquiry

137862-53-4
905 suppliers
$9.00/10g
![L-Valine, N-[[2'-(2H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-N-(2-thienylcarbonyl)-](/CAS/20210305/GIF/1033772-60-9.gif)
1033772-60-9
0 suppliers
inquiry

137862-53-4
905 suppliers
$9.00/10g
![N-[2’-(1H-tetrazol-5-yl)biphenyl-4-yl methyl]-N-Valeryl-(L)-Valine benzyl ester](/CAS/GIF/137863-20-8.gif)
137863-20-8
109 suppliers
$70.00/5mg

137862-53-4
905 suppliers
$9.00/10g