
Omeprazole synthesis
- Product Name:Omeprazole
- CAS Number:73590-58-6
- Molecular formula:C17H19N3O3S
- Molecular Weight:345.42
Synthesis and Structure of Omeprazole
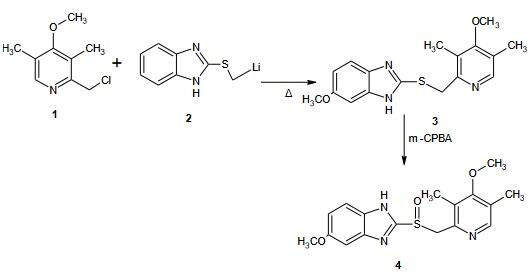
Steps: 2-(Lithium methyl sulphinyl)-5-methoxy-1H benzimidazole 20g was reacted with 2-chloro-3,5-dimethyl-4-methoxy pyridine 21 g to form sulphide intermediate and then converted to Omeprazole when treated with m-CPBA which used as anoxidizingagents. The acetamide-sulfide compounds modification are oxidised to form the amide sulfinyl compound and gives the sulfinyl carboxylate or salts upon alkaline hydrolysis.On further decarboxylation leads to the target molecules. The residual, unreacted salt, inorganic by-products and other minor by-products can be easily purified by a simple washing from omeprazole or lansoprazole. The amide compounds containing crystalline solids as opposed to the sulphide and sulfoxides of the reported procedures.
DOI: http://dx.doi.org/10.20902/IJPTR.2019.120307
86604-75-3
571 suppliers
$10.00/1g
37052-78-1
648 suppliers
$11.00/25g

73590-58-6
708 suppliers
$5.00/100mg
Yield:73590-58-6 96%
Reaction Conditions:
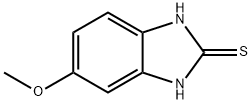
Stage #1: 6-methoxy-1H-benzoimidazole-2-thiolwith sodium hydroxide in ethanol;water at 70 - 90;
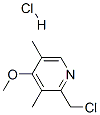
Stage #2: 2-(chloromethyl)-4-methoxy-3,5-dimethylpyridine hydrochloridewith hydrogenchloride in ethanol;water at -10 - 30; for 4 h;Reflux;
Steps:
11 Preparation of 5-methoxy-2- (4-methoxy-3,5-dimethyl-2-pyridyl) methylthio-1H-benzimidazole
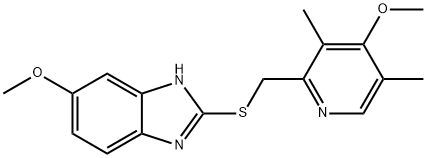
In a 1000 mL three-necked flask,Sodium hydroxide (5 g, 0.13 mol) was added to ethanol (50 mL) and heated to 70-90After dissolving, 2-mercapto-5-methoxybenzimidazole (17.8 g, 0.10 mol) was added,Reflux dissolved,Cooling to below 10 .2-chloromethyl-4-methoxy-3,5-dimethylpyridine hydrochloride (20 g, 0.09 mol) was dissolved in a 200 mL constant pressure dropping funnelIn water (100 mL), the aqueous solution of hydrochloric acid was slowly added dropwise, and the temperature was raised to 30 ° C. After the incubation reaction was carried out for 4 hours,Cooling to 10 ,500 mL of water was added and stirred for 12 hours. A white solid was obtained by suction filtration and dried to give 5-methoxy-2- (4-methoxy-3,5-dimethylYl-2-pyridyl) methylthio-1H-benzimidazole in a yield of 96%.
References:
CN107011252,2017,A Location in patent:Paragraph 0084; 0085
73590-85-9
473 suppliers
$30.00/10mg

73590-58-6
708 suppliers
$5.00/100mg
1080503-61-2
0 suppliers
inquiry
109371-19-9
63 suppliers
inquiry

73590-58-6
708 suppliers
$5.00/100mg
![5-Methoxy-2-[[(4-Methoxy-3,5-diMethyl-2-pyridinyl)Methyl]thio]-1H-benziMidazole N-Oxide](/CAS/20150408/GIF/142885-92-5.gif)
142885-92-5
59 suppliers
inquiry

73590-58-6
708 suppliers
$5.00/100mg
408332-88-7
4 suppliers
inquiry

73590-85-9
473 suppliers
$30.00/10mg

73590-58-6
708 suppliers
$5.00/100mg