
Dimethyl sulfoxide synthesis
- Product Name:Dimethyl sulfoxide
- CAS Number:67-68-5
- Molecular formula:C2H6OS
- Molecular Weight:78.13

75-18-3
396 suppliers
$19.00/25mL

67-68-5
1243 suppliers
$15.00/100g
Yield:67-68-5 100%
Reaction Conditions:
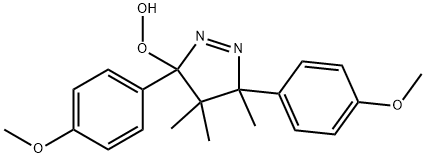
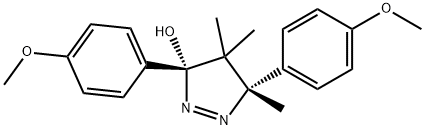
with C12H11Cl3N2O3 in chloroform; for 7 h;Reflux;
Steps:
Oxygen transfer to sulfides with oxaziridines in the absence of acid
General procedure: A solution of oxaziridine 3, 4, 1b, and 1c (1 mmol) in chloroform (20 mL) was heated at reflux in the presence of sulfide (1 mmol). The reaction was taken under magnetic stirring and heating for 7h and monitored by TLC. Then, the reaction products were identified by 1H NMR spectroscopic analysis. All the isolated sulfoxide compounds were purified by chromatography over silica gel using dichloromethane/methanol 95:5 as eluent. The sulfoxides obtained are compared and identified with commercial samples. There sults obtained are presented in Table 1 (entries 2, 5, and 6). This reaction was carried out using also acetonitrile (20 mL) and methanol (20 mL) as solvent. There sults obtained are presented in Table 1 (entries 3and 4).
References:
Aydi, Rihab;Kammoun, Majed [Synthetic Communications,2016,vol. 46,# 2,p. 134 - 144]

75-18-3
396 suppliers
$19.00/25mL
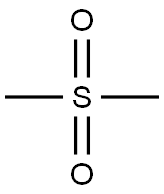
67-71-0
581 suppliers
$10.00/10g

67-68-5
1243 suppliers
$15.00/100g
![Ruthenium, dichloro[[2-[(diphenylarsino)methyl]-2-methyl-1,3-propanediyl]bis[diphenylarsine]-As,As',As''][sulfinylbis[methane]-O]-, (OC-6-32)- (9CI)](/CAS/20211123/GIF/112220-32-3.gif)
![Ruthenium, carbonyldichloro[[2-[(diphenylarsino)methyl]-2-methyl-1,3-propanediyl]bis[diphenylarsine]-As,As',As'']-, (OC-6-32)- (9CI)](/CAS/20211123/GIF/112220-42-5.gif)