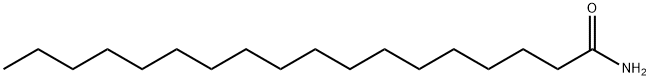
Octadecanamide synthesis
- Product Name:Octadecanamide
- CAS Number:124-26-5
- Molecular formula:C18H37NO
- Molecular Weight:283.49

57-11-4
1231 suppliers
$6.00/100g

124-26-5
315 suppliers
$14.00/100g
Yield:124-26-5 98.4%
Reaction Conditions:
with titanium(IV) isopropylate;ammonia at 165; for 7 h;Large scale;Reagent/catalyst;Temperature;
Steps:
1
According to the embodiment of the present invention described above, stearic acid amide, which is a kind of carboxylic acid amide compound, was prepared in Example 1 as follows. First, the carboxylic acid injector 250 injects 1000 g of stearic acid into the heater 100, and the heater 100 heats 1000 g of stearic acid to 120 ° C. Subsequently, when stearic acid is injected into the first reaction tank 210, the first catalyst injector 261 injects 10 g of tetraisopropyl titanium, which is a metal catalyst, into the first reaction tank 210, Was heated by the heater attached to the first reaction tank 210. When 150 g of stearic acid was charged into the first reaction tank 210, the first ammonia injector 281 started to feed the ammonia gas through the ammonia pipe 283 at a rate of 100 L / hr. When 500 g of stearic acid was charged into the first reaction tank 210, the introduction of stearic acid into the first reaction tank 210 was stopped. The propeller in the first reaction tank 210 was mixed with stearic acid and ammonia while maintaining the reaction temperature at 165 ° C in the first reaction tank 210.Next, when the supply of the stearic acid to the first reaction tank 210 is stopped, the 500 g of stearic acid remaining in the heater 100 through the valve is changed to be supplied to the second reaction tank 220. When stearic acid is injected into the second reaction tank 220, the second catalyst injector 262 injects 10 g of tetraisopropyl titanium as a metal catalyst into the second reaction tank 220 and starts heating the second reaction tank 220 . When 150 g of stearic acid was charged into the second reaction tank 220, the second ammonia feeder 282 started to feed the ammonia gas through the ammonia pipe 283 at a rate of 100 L / hr. When all 500 g of stearic acid was fed to the second reaction tank, the addition of stearic acid to the second reaction tank was stopped. The propeller in the second reaction tank 220 was mixed with stearic acid and ammonia while maintaining the reaction temperature of 165 ° C in the second reaction tank 220.
References:
Lee, Chang Ho KR101678461, 2016, B1 Location in patent:Paragraph 0059-0061; 0068

112-76-5
244 suppliers
$40.32/25G

124-26-5
315 suppliers
$14.00/100g

638-65-3
57 suppliers
$54.00/5g

124-26-5
315 suppliers
$14.00/100g

124-30-1
402 suppliers
$8.00/25g

124-26-5
315 suppliers
$14.00/100g
