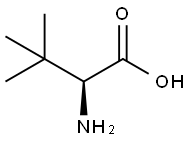
L-tert-Leucine synthesis
- Product Name:L-tert-Leucine
- CAS Number:20859-02-3
- Molecular formula:C6H13NO2
- Molecular Weight:131.17
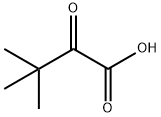
815-17-8
285 suppliers
$41.00/25g

20859-02-3
603 suppliers
$9.00/5G
Yield:20859-02-3 80%
Reaction Conditions:
with ammonium hydroxide;D-glucose;ammonium chloride;sodium hydroxide in water; pH=8.3; for 5.5 h;Enzymatic reaction;stereoselective reaction;Time;Reagent/catalyst;
Steps:
2 2.8 Reductive amination of TMP to l-tert-leucine in liter-scale
Bioconversion of TMP to l-tert-leucine was carried out by recombinant whole-cells harboring pET28a-E-G. In a reactor (2L), 1L of water was added to a mixture of 178.2g glucose (0.9mol), 78.1g TMP (0.6mol), 26.8g NH4Cl (0.5mol) and 10.0g of the whole cells of E. coli BL21/pET28a-E-G. The reaction pH was adjusted to 8.5 with NaOH solution. During the reaction process, the pH was automatically adjusted to 8.5 by titrating 50% (v/v) NH3·H2O. When the reaction was terminated, the reaction mixture was heated to 60°C, followed by centrifugation to remove the biocatalysts. The remained solution was adjusted to pH 5.9 and concentrated via rotary evaporator. Then the solution temperature was decreased slowly to 25°C and continued to decrease to 4°C, the l-tert-leucine crystals were obtained.
References:
Li, Jing;Pan, Jiang;Zhang, Jie;Xu, Jian-He [Journal of Molecular Catalysis B: Enzymatic,2014,vol. 105,p. 11 - 17]
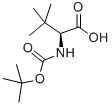
62965-35-9
444 suppliers
$6.00/5g

20859-02-3
603 suppliers
$9.00/5G
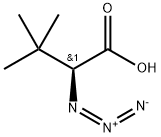
111525-71-4
2 suppliers
inquiry

20859-02-3
603 suppliers
$9.00/5G
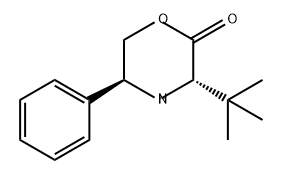
202347-81-7
0 suppliers
inquiry

20859-02-3
603 suppliers
$9.00/5G
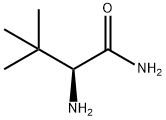
62965-57-5
13 suppliers
inquiry

20859-02-3
603 suppliers
$9.00/5G