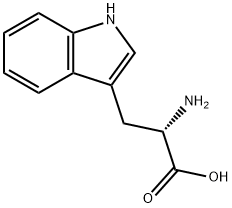
L-Tryptophan synthesis
- Product Name:L-Tryptophan
- CAS Number:73-22-3
- Molecular formula:C11H12N2O2
- Molecular Weight:204.23
However, the manufacturer using genetically modified strains derived fromBacillus amyloliquefaciens IAM 1521 was forced to stop Ltryptophan production. L-Tryptophan produced by this process was stigmatized because of side products found in the product causing a new severe disease termed eosinophilia-myalgia syndrome (EMS). One of the problematic impurities, "Peak E", was identified as 1,10-ethylidene- bis-(L-tryptophan), a product formed by condensation of one molecule acetaldehyde with two molecules of tryptophan.

1218-34-4
391 suppliers
$10.00/1g

73-22-3
976 suppliers
$5.00/250mg
Yield:73-22-3 98%
Reaction Conditions:
with pepsin immobilized on terephthalaldehyde functionalized chitosan magnetic nanoparticle in acetonitrile at 20; pH=2; for 48 h;
Steps:
Deacetylation of Amides
General procedure: To a solution of an amide (1 mmol) in 10 mL of acetonitrile and 10 mLof citrate buffer (pH = 2.0) was added the catalyst (100 mg). The reactionmixture was stirred at rt, and the progress of the reaction was monitored byTLC using ethyl acetate/n-hexane 50:50 as the eluent. After 48 h, the catalystswere removed and washed with HCl (0.01M). The crude residue was purifiedby preparative silica gel plate chromatography using ethyl acetate/n-hexane 50:50 as the eluent. The recovered catalyst was used for the next reaction cycleby the same procedure described above.
References:
Lakouraj, Moslem Mansour;Maashsani, Azita;Norouzian, Rafieh-Sadat;Mohadjerani, Maryam [Journal of Carbohydrate Chemistry,2015,vol. 34,# 2,p. 103 - 119]
![L-Tryptophan, N,1-bis[(1,1-dimethylethoxy)carbonyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/176536-74-6.gif)
176536-74-6
0 suppliers
inquiry

73-22-3
976 suppliers
$5.00/250mg
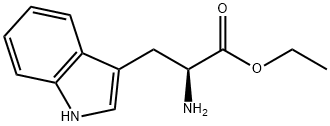
7479-05-2
14 suppliers
inquiry

73-22-3
976 suppliers
$5.00/250mg
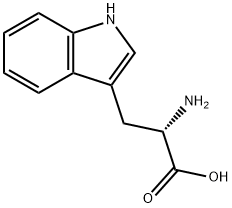
54-12-6
372 suppliers
$6.00/5g

73-22-3
976 suppliers
$5.00/250mg
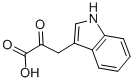
392-12-1
174 suppliers
$85.00/1g

73-22-3
976 suppliers
$5.00/250mg