L-Serine synthesis
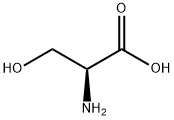
- Product Name:L-Serine
- CAS Number:56-45-1
- Molecular formula:C3H7NO3
- Molecular Weight:105.09
The biosynthesis of serine starts with the oxidation of 3- phosphoglycerate to 3-phosphohydroxypyruvate and NADH. Reductive amination of this ketone followed by hydrolysis gives serine. Serine hydroxymethyltransferase catalyzes the reversible, simultaneous conversions of L-serine to glycine (retro-aldol cleavage) and 5,6,7,8-tetra hydrofolate to 5,10-methylene tetra hydrofolate (hydrolysis).
This compound may also be naturally produced when UV light illuminates simple ices such as a combination of water, methanol, hydrogen cyanide, and ammonia, suggesting that it may be easily produced in cold regions of space.
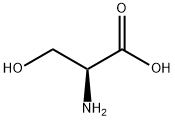
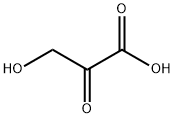
302-84-1
537 suppliers
$5.00/25g

56-45-1
764 suppliers
$5.00/25g
Yield:99.4 % ee
Reaction Conditions:
in methanol;water at 39.99; for 48 h;Purification / work up;
Steps:
1
A physical mixture of crystals of the racemic compound and the L-enantiomer (53.60 g and 13.19 g) were added to a 300 ml vessel. The mixture was enriched by the target enantiomer (optical purity 59.9%). A solvent consisting of 81.01 g water and 121.52 g methanol (60:40 wt/wt) was added and the slurry was properly agitated and kept at isothermal conditions at 313.15 K for 2 days to ensure thermodynamic solid/liquid phase equilibrium. The overall composition was chosen to represent an output of a previous partial enrichment step by chiral chromatography e.g. on a SMB system. Analysis by means of chiral chromatography yielded an optical purity in the liquid phase of 99.4% L-enantiomer after equilibration.The liquid phase was removed through a filter and dried to dryness. The generated crystals were of 99.4% optical purity.
References:
US2011/263896,2011,A1 Location in patent:Page/Page column 3-4
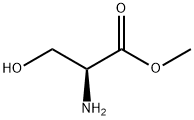
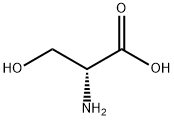
2788-84-3
28 suppliers
$367.00/0.25G

56-45-1
764 suppliers
$5.00/25g
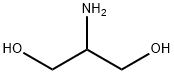
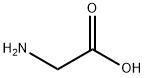
534-03-2
464 suppliers
$7.00/10g
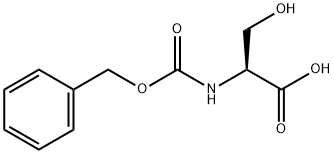
1145-80-8
333 suppliers
$5.00/5g

56-45-1
764 suppliers
$5.00/25g