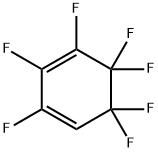
hexafluorobenzene synthesis
- Product Name:hexafluorobenzene
- CAS Number:392-56-3
- Molecular formula:C6F6
- Molecular Weight:186.05
C6Cl6 + 6 KF → C6F6 + 6 KCl
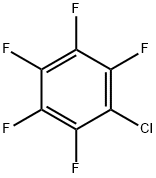
344-07-0
192 suppliers
$10.00/2 g

392-56-3
297 suppliers
$9.00/1g
Yield:392-56-3 71.7%
Reaction Conditions:
with sulfolane;potassium fluoride;tetrakis(diethylamino)phosphonium bromide in nitrobenzene;toluene at 120 - 240; for 30 h;Inert atmosphere;Autoclave;Reagent/catalyst;Temperature;
Steps:
2 Example 2
In a 1000 ml four-necked flask equipped with a water separator, 134 g of potassium fluoride, 350 ml of sulforan and 300 ml of toluene were successively added. After stirring under mechanical stirring, the mixture was heated to 120-130°C under reflux, and the water was separated. After no water was removed, the water separator was turned on and the heat was removed to remove the toluene at 185°C. When no toluene was distilled off, the nitrogen protection was allowed to cool to room temperature, transferred to a 1000 ml autoclave, 314 g of chloropentafluorobenzene, 15 g of tetrakis(diethylamino)phosphonium bromide, and 5 g of nitrobenzene were added, and the temperature was raised to 240°C for 30 hours. . After the reaction was completed, the reaction system was distilled at atmospheric pressure and received a fraction of 40 to 160°C. The above-mentioned receiver was subjected to atmospheric rectification using a 30 cm packed distillation column, and at the same time, a 79-81° C. fraction was received to obtain a final product with a purity of more than 99%. The yield was 71.7%.
References:
Dalian Qikai Pharmaceutical Technology Co., Ltd.;Tian Hanqing;Jiang Dianping;Zhang Hongxue CN107827704, 2018, A Location in patent:Paragraph 0024-0031
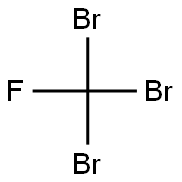
353-54-8
92 suppliers
$51.00/5g

392-56-3
297 suppliers
$9.00/1g
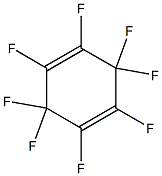
775-51-9
1 suppliers
inquiry

392-56-3
297 suppliers
$9.00/1g
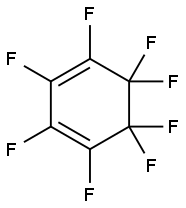
377-70-8
0 suppliers
inquiry

392-56-3
297 suppliers
$9.00/1g