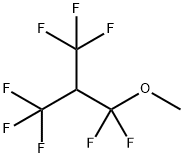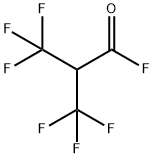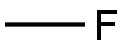
METHYL FLUORIDE synthesis
- Product Name:METHYL FLUORIDE
- CAS Number:593-53-3
- Molecular formula:CH3F
- Molecular Weight:34.03

77-78-1
303 suppliers
$19.69/1ml
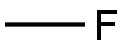
593-53-3
71 suppliers
inquiry
Yield:593-53-3 100%
Reaction Conditions:
with potassium fluoride in water at 100; for 5 h;Solvent;Reagent/catalyst;Temperature;
Steps:
1 i.i Example 1
After i 2 g (0.2 i mol) ofpotassium fluoride and 25 g of pure water were placed in a i 00-cc pressure-resistant container, i 0 g (0.08 mol) of dimethyl sulfate was added to start the reaction. Afier the temperature had reached iOO° C., the reaction was allowed to proceed for 5 hours. The final pressure was 0. iS MPa/G. The generated gas was collected and analyzed by gas chromatography. With the theoretical yield of methane fluoride obtained by this reaction being assumed to be 0.08 mol, the yield of methane fluoride was calculated and found to be 0.08 mol. The yield was iOO%.
References:
DAIKIN INDUSTRIES, LTD.;NAKAMURA, Shingo;FUKUMOTO, Kanako;ETOU, Yuusuke;OHTSUKA, Tatsuya;HIGASHI, Masahiro US2016/168060, 2016, A1 Location in patent:Paragraph 0053

74-87-3
210 suppliers
$18.50/48622
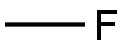
593-53-3
71 suppliers
inquiry

74-88-4
349 suppliers
$15.00/10g
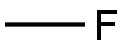
593-53-3
71 suppliers
inquiry
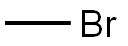
74-83-9
2 suppliers
$24.10/48624
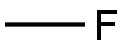
593-53-3
71 suppliers
inquiry