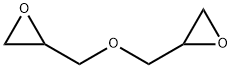
DIGLYCIDYL ETHER synthesis
- Product Name:DIGLYCIDYL ETHER
- CAS Number:2238-07-5
- Molecular formula:C6H10O3
- Molecular Weight:130.14

702-23-8
150 suppliers
$9.00/5g

106-89-8
753 suppliers
$9.00/10 g

2238-07-5
36 suppliers
$47.75/50mg
Yield:2238-07-5 67%
Reaction Conditions:
with Bu4NHSO4
Steps:
24.1 N-Methyl-N-[1-[2-hydroxy-3-[2-(4-methoxyphenyl)ethoxy]-propyl]-4-piperidyl]-4H-3,1-benzothiazin-2-amine Hydrogen Fumarate (24)
24.1) 1-[2-(4-methoxyphenyl)ethoxy]-2,3-epoxypropane (24.1). By condensation of 4.8 g (31.5 mmol) of 4-methoxyphenethyl alcohol in a mixture of 25 ml of epichlorohydrin and 18 ml of 50% caustic soda and 430 mg of Bu4NHSO4 according to process 23.1, 4.42 g (yield: 67%) of glycidyl ether of formula 24.1 are prepared: Empirical formula: C12H16O3; Molecular mass: 208.25; Colorless oil; Boiling point: 130-135° C./0.25 mbar; NMR (CDCl3) δ: 2.6 (s, 1H); 2.78 (s, 1H); 2.79-2.91 (m, 2H); 3 (s, 1H); 3.34-3.5 (m, 1H); 3.5-3.89 (m, 3H); 3.9; (s, 3H); 6.83 (d, 2H); 7.14 (d, 2H).
References:
Pierre Fabre Medicament US6531469, 2003, B1
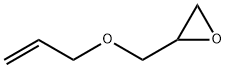
106-92-3
283 suppliers
$14.00/5mL

2238-07-5
36 suppliers
$47.75/50mg

106-89-8
753 suppliers
$9.00/10 g

2238-07-5
36 suppliers
$47.75/50mg
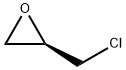
51594-55-9
365 suppliers
$10.00/1g

2238-07-5
36 suppliers
$47.75/50mg
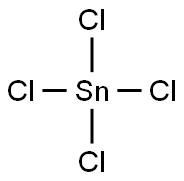
7646-78-8
306 suppliers
$11.00/5g

106-89-8
753 suppliers
$9.00/10 g

2238-07-5
36 suppliers
$47.75/50mg