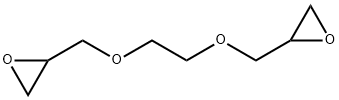
Ethylene glycol diglycidyl ether synthesis
- Product Name:Ethylene glycol diglycidyl ether
- CAS Number:2224-15-9
- Molecular formula:C8H14O4
- Molecular Weight:174.19

107-21-1
1335 suppliers
$10.00/25g

106-89-8
752 suppliers
$9.00/10 g

2224-15-9
258 suppliers
$23.00/25g
Yield:2224-15-9 89%
Reaction Conditions:
with tetrabutyl-ammonium chloride;water;sodium hydroxide at 40; for 0.75 h;
Steps:
2.3. General method for synthesis of diglycidyl ether intermediates
General procedure: Diglycidyl ether intermediates were prepared under phase-transfercatalytic condition: To the vigorously stirred mixture of epichlorohydrin(9.34 mL, 120 mmol), sodium hydroxide pellets (4.8 g,120 mmol), water (0.5 mL, 28 mmol), and tetrabutylammoniumchloride (0.28 g, 1 mmol), cooled Glycol (20 mmol, 1,2-ethanediol or1,4-butanediol) was added dropwise. After the completion of the addition,the mixture was stirred for 45 min at 40 °C. The solid productsthat were formed alongside the reaction removed by filtration andwashed with CH2Cl2 (30 mL×3). The combined organic layers weredried over anhydrous Na2SO4 and additional dichloromethane andexcess epichlorohydrin evaporated off by distillation to give a yellowoil. The resulting oil was purified by distillation under reduced pressureto give a pure and colorless product (89% yields). 2.3.1. 1,2-Ethanediol diglycidyl ether (EDDGE)1H NMR (400 MHz, CDCl3): δ 3.59-3.71 (m, 4H, OCH2CH2),3.77-3.80 (dd, 2H, OCH2), 3.38-3.42 (dd, 2H, OCH2), 3.13-3.16 (m,2H, CH2(O)CH), 2.58-2.60 (dd, 2H, CH(O)CH2), 2.76-2.78 (dd, 2H, CH(O)CH2).
References:
Abbasi, Hassan;Pourrostam-Ravadanaq, Pariya;Safa, Kazem D. [Analytical Biochemistry,2020,vol. 597]
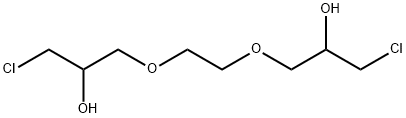
13078-45-0
2 suppliers
inquiry

2224-15-9
258 suppliers
$23.00/25g