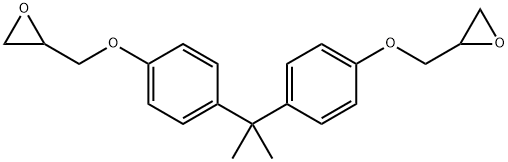
BISPHENOL A DIGLYCIDYL ETHER RESIN synthesis
- Product Name:BISPHENOL A DIGLYCIDYL ETHER RESIN
- CAS Number:1675-54-3
- Molecular formula:C21H24O4
- Molecular Weight:340.41
80-05-7
666 suppliers
$13.00/100g

106-89-8
746 suppliers
$9.00/10 g

1675-54-3
182 suppliers
$9.00/25g
Yield:1675-54-3 72%
Reaction Conditions:
Stage #1:BPA with sodium hydride in N,N-dimethyl-formamide at 20; for 0.25 h;Inert atmosphere;
Stage #2:epichlorohydrin in N,N-dimethyl-formamide at 20; for 18 h;
Steps:
7
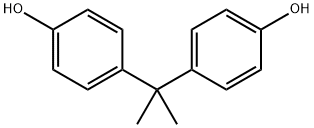
A round-bottomed flask was charged sequentially with NaH (200 mg, 4.80 mmol, 2.2 equiv) and bisphenol A (500 mg, 2.18 mmol, 1 equiv), and the contents were placed under an atmosphere of argon. Anhydrous dimethyl formamide (5 mL) was introduced via syringe and the resulting mixture was stirred at room temperature. After 15 min, racemic epichlorohydrin (700 μL, 8.96 mmol, 4.1 equiv) was added via syringe and the mixture was allowed to react at room temperature for 18 h. Then, the solution was quenched with deionized water (~ 1 mL) and the mixture was extracted with ethyl acetate (3 x 4 mL). The organic layer was washed with deionized water (2 mL), was dried over anhydrous magnesium sulfate, was filtered, and was concentrated under reduced pressure. The resulting residue was purified by flash column chromatography on silica gel (eluent: dichloromethane) to provide racemic BADGE (536 mg, 72%) as a white foamy residue. 1H NMR (400 MHz, DMS(W6): δ 7.10 (d, J= 8.8, 4H), 6.84 (d, J= 8.8, 4H), 4.25 (dd, J = 11.6, 2.8, 2H), 3.78 (dd, J= 11.2, 6.4, 2H), 3.29 (m, 2H), 2.81 (t, J= 4.8, 2H), 2.68 (m, 2H), 1.60 (s, 6H). 13C NMR (100 MHz, DMSO-J6): δ 156.6, 143.5, 128.0, 114.5, 69.5, 50.3, 44.4, 41.8, 31.3. TLC (5% methanol in dichloromethane), R/ 0.77 (UV, p- anisaldehyde).
References:
BRITISH COLUMBIA CANCER AGENCY BRANCH;THE UNIVERSITY OF BRITISH COLUMBIA;SADAR, Marianne, D.;MAWJI, Nasrin, R.;WANG, Jun;ANDERSEN, Raymond, J.;WILLIAMS, David, E.;LEBLANC, Mike WO2010/66, 2010, A1 Location in patent:Page/Page column 77-78