
Cyclobutanone synthesis
- Product Name:Cyclobutanone
- CAS Number:1191-95-3
- Molecular formula:C4H6O
- Molecular Weight:70.09
Another synthesis of cyclobutanone involves lithium-catalyzed rearrangement of oxaspiropentane, which is formed by epoxidation of the easily accessible methylenecyclopropane.
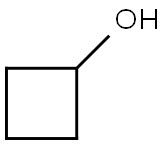
2919-23-5
183 suppliers
$9.00/1g

1191-95-3
325 suppliers
$10.00/1g
Yield:1191-95-3 99.2%
Reaction Conditions:
with sodium chlorine monoxide;2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;Sodium hydrogenocarbonate in ethyl acetate at 10; for 4 h;
Steps:
3 The third reaction step:
Add the EA phase containing product A4 in the previous step to the dry 2L four-neck reaction flask, add 500g NaHCO3 and 10g TEMPO;The temperature of the ice-water bath was controlled to below 10°C, and a 10% aqueous solution of sodium hypochlorite was added in batches. During the addition, the system exotherm was obvious, and with the outgassing, after 4 hours of reaction, the original A4 of GC remained 9%, and NaHCO3 and TEMPO were added. After 10 hours, the original reaction of GC was complete. .Post-processing:The reaction system was suction filtered at a low temperature, and the filter cake was rinsed with a small amount of EA. The filtrate was light yellow. A small amount of sodium thiosulfate solid was added and stirred for 2h. The system became dark brown. 100g of sodium bicarbonate was added and stirred for 1h. Sodium sulfate is dried; filtered, the filtrate is equipped with a thorn column, and most of the solvent is distilled off at atmospheric pressure, and then changed to vacuum distillation for distillation, to obtain 44g of product A5, and the gas phase shows the purity of the product is 99.2%.
References:
CN111138252,2020,A Location in patent:Paragraph 0015; 0019; 0020
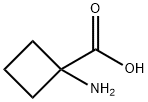
22264-50-2
282 suppliers
$11.00/1g

1191-95-3
325 suppliers
$10.00/1g
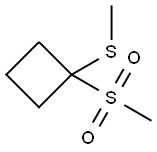
78787-13-0
0 suppliers
inquiry

1191-95-3
325 suppliers
$10.00/1g
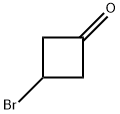
23761-24-2
87 suppliers
$30.00/100mg

1191-95-3
325 suppliers
$10.00/1g
1120-56-5
37 suppliers
$163.00/1g

1191-95-3
325 suppliers
$10.00/1g