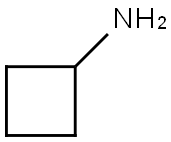
Cyclobutylamine synthesis
- Product Name:Cyclobutylamine
- CAS Number:2516-34-9
- Molecular formula:C4H9N
- Molecular Weight:71.12
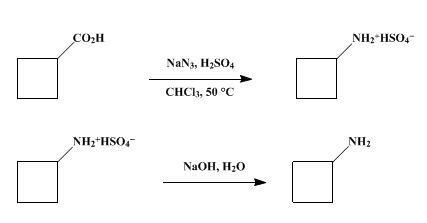
Synthesis of cyclobutylamine

1191-95-3
329 suppliers
$10.00/1g

2516-34-9
232 suppliers
$25.00/1g
Yield:2516-34-9 72%
Reaction Conditions:
Stage #1: cyclobutanonewith palladium 10% on activated carbon;benzylamine in toluene at 40; for 10 h;Autoclave;
Stage #2: with hydrogen in toluene at 40 - 80; under 22801.5 Torr; for 24 h;Autoclave;Reagent/catalyst;Pressure;Temperature;Solvent;
Steps:
1-3 Example 3
In a 100 ml autoclave, 10 g of cyclobutanone, 20 g of benzylamine,20 grams of toluene and 0.2 grams of palladium on carbon, heated to 40 ° C for 10 hours;Introduce hydrogen into the autoclave, and keep the pressure of hydrogen at 30atm.Continue to raise the temperature to 80 ° C and react for 24 hours. The gas chromatograph monitors the disappearance of cyclobutanone.The reaction was completed, the palladium carbon was removed by filtration, and the filtrate was rectified to collect the fraction having a boiling point of 85-86 ° C.7.2 g of cyclobutylamine were obtained with a yield of 72% and a purity of 98.0%.
References:
CN110483306,2019,A Location in patent:Paragraph 0025-0027
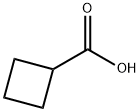
3721-95-7
417 suppliers
$7.00/5g

2516-34-9
232 suppliers
$25.00/1g
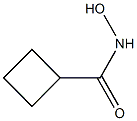
77317-97-6
4 suppliers
inquiry

2516-34-9
232 suppliers
$25.00/1g

2625-41-4
45 suppliers
inquiry

2516-34-9
232 suppliers
$25.00/1g
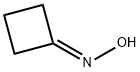
2972-05-6
22 suppliers
$150.00/1g

2516-34-9
232 suppliers
$25.00/1g