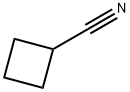
Cyclobutanecarbonitrile synthesis
- Product Name:Cyclobutanecarbonitrile
- CAS Number:4426-11-3
- Molecular formula:C5H7N
- Molecular Weight:81.12

1189-71-5
343 suppliers
$18.00/5g
3721-95-7
412 suppliers
$7.00/5g

4426-11-3
122 suppliers
$25.00/1g
Yield:4426-11-3 66.5%
Reaction Conditions:
in dichloromethane;N,N-dimethyl-formamide
Steps:
27.a a
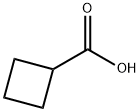
a Cyclobutanecarbonitrile A solution of 80 g (799 mmoles) of cyclobutanecarboxylic acid in 250 ml of dichloromethane is refluxed. 115.6 g (819 mmoles) of chlorosulfonylisocyanate are poured slowly dropwise. Reflux is continued one hour after the end of the addition, until complete evolution of CO2. The reaction mixture is then cooled to 10° C. During 15 min., 119.6 g (1638 mmoles) of N,N-dimethylformamide are poured dropwise therein, before allowing stirring 1/4 hour at room temperature. Then, the mixture is poured over ice-water, before extracting it with dichloromethane. The organic phase washed with water, is dried over Na2 SO4, and concentrated under vacuum. The distillation of the residue gives 43.5 g (yield=66.5%) of a colorless liquid. b.p.15 =50° C. I.R. (film): ν (C N)=2250 cm-1. N.M.R. (CDCl3): δ=1.5-2.7 (6H,m); 2.7-3.4 (1H,m).
References:
Lipha Lyonnaise Industrielle Pharmaceutique US5387709, 1995, A

1503-98-6
85 suppliers
$29.00/100mg

4426-11-3
122 suppliers
$25.00/1g
6280-87-1
218 suppliers
$10.00/1g

4426-11-3
122 suppliers
$25.00/1g

5414-21-1
156 suppliers
$10.00/5g

4426-11-3
122 suppliers
$25.00/1g

3721-95-7
412 suppliers
$7.00/5g

4426-11-3
122 suppliers
$25.00/1g