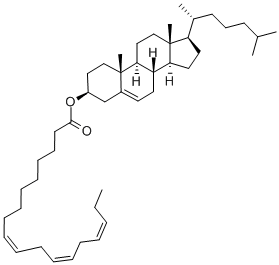
Cholesteryl linolenate synthesis
- Product Name:Cholesteryl linolenate
- CAS Number:2545-22-4
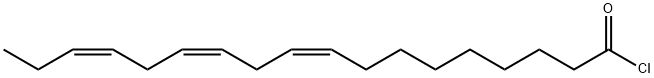
- Molecular formula:C45H74O2
- Molecular Weight:647.07
Yield:-
Reaction Conditions:
in benzene at 20;
Steps:
Synthesis of Cholesteryl Esters
General procedure: The recovery standard pentadecanoic acid cholesteryl ester(hereafter referred to as 15:0-CE) was prepared accordingto Lusby et al. [20] with slight modifications. Pentadecanoicacid (121 mg, 0.5 mmol) was placed in a 50-ml flaskand dissolved in 10 ml of toluene. About 120 μl thionylchloride was added, and the solution was refluxed for 4 hto obtain the acid chloride. Afterwards, the remaining thionylchloride and parts of the toluene were removed underreduced pressure. To the residue (about 2 ml), 153 mgcholesterol (0.4 mmol) dissolved in 10 ml toluene wasadded, and the solution was stirred overnight at room temperature.The solvent was removed on a rotary evaporator,and the residue was re-dissolved in 4 ml n-hexane. Thissolution was purified by SPE. For this purpose, 15 g ofsilica gel deactivated with 20 % water (w/w) was giveninto a glass column (i.d. 2.5 cm) and pre-conditionedwith n-hexane. After 50 ml of n-hexane (fraction 1), three50-ml fractions (fractions 2-4) were collected with n-hexane/ethyl acetate (99:1, v/v) as eluent. Fractions 3 and 4yielded 154.3 and 64.8 mg of 15:0-CE. The purity inboth fractions was >99 % according to GC/MS and NMRspectroscopy analysis
References:
Hammann, Simon;Wendlinger, Christine;Vetter, Walter [Lipids,2015,vol. 50,# 6,p. 611 - 620]