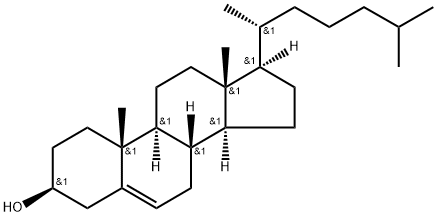
Cholesterol synthesis
- Product Name:Cholesterol
- CAS Number:57-88-5
- Molecular formula:C27H46O
- Molecular Weight:386.66
The commercial material is normally obtained from the spinal cord
of cattle by extraction with petroleum ethers, but it may also be
obtained from wool fat. Purification is normally accomplished by
repeated bromination. Cholesterol may also be produced by entirely
synthetic means.
Cholesterol produced from animal organs will always contain
cholestanol and other saturated sterols.
Cellular cholesterol derives from two main sources: Dietary cholesterol circulates in high- and low-density lipoproteins (HDL and LDL), and can be directly taken up by cells for processing in the lysosome. Alternatively, cholesterol can be synthesised within cells from acetyl-CoA. This occurs within the endoplasmic reticulum (ER) membrane, and involves a series of enzymatic steps governed by activation of the sterol response element binding protein 2 (SREBP2) transcription factor, and stabilisation of 3-Hydroxy-3-Methylglutaryl-CoA Reductase (HMGCR), the rate-limiting enzyme in cholesterol synthesis. 7-dehydrocholesterol (7DHC) is the immediate precursor of cholesterol.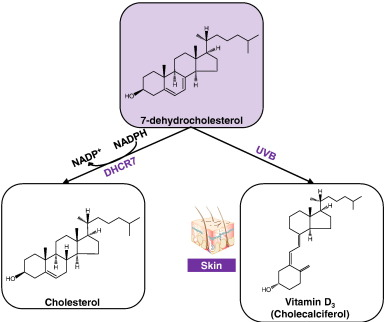
604-35-3
242 suppliers
$52.39/25g

57-88-5
792 suppliers
$10.00/1g
Yield:57-88-5 99.1%
Reaction Conditions:
with methanol;potassium carbonate at 65; for 2 h;Inert atmosphere;
Steps:
7.1 Preparation of cholesterol by hydrolysis of compound of formula (7-1)
Methanol (40 mL) and K2CO3 (1.28 g, 12.1 mmol) were added to the flask,After dissolving, the compound of formula (7-1) (4g, 9.33mmol) was added under the protection of N2,The temperature was raised to 65°C and reacted for 2h. After monitoring the reaction by TLC, it was cooled to 25°C.Add 2 mol/L dilute hydrochloric acid to adjust PH=7-8, evaporate methanol under reduced pressure, add water (20 mL), stir at 25 °C for 2 h, and filter with suction; add water (20 mL) to the filter cake, stir at 25 °C for 2 h, and filter with suction ,Dry to obtain refined cholesterol (CCDC 2099442, single crystal structure is shown in Figure 8,White solid 3.57g, molar yield 99.1%, gas chromatography purity 99.10%, see Figure 9).
References:
CN114395009,2022,A Location in patent:Paragraph 0208-0211
57711-50-9
7 suppliers
$165.00/10mg

57-88-5
792 suppliers
$10.00/1g
1249-67-8
19 suppliers
inquiry

57-88-5
792 suppliers
$10.00/1g
![Cholest-5-ene, 3-[(4-methoxyphenyl)methoxy]-, (3β)-](/CAS/20210305/GIF/33999-75-6.gif)
33999-75-6
0 suppliers
inquiry

57-88-5
792 suppliers
$10.00/1g
604-32-0
200 suppliers
$28.60/10MG

57-88-5
792 suppliers
$10.00/1g