Bendamustine hydrochloride synthesis
- Product Name:Bendamustine hydrochloride
- CAS Number:3543-75-7
- Molecular formula:C16H22Cl3N3O2
- Molecular Weight:394.72
![5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid ethyl ester](/CAS/GIF/3543-74-6.gif)
3543-74-6
190 suppliers
$25.00/250mg

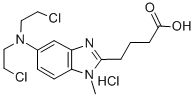
3543-75-7
376 suppliers
$35.00/10 mg
Yield:3543-75-7 76.2%
Reaction Conditions:
Stage #1:4-{5-[bis-(2-hydroxyl-ethyl)-amino]-1-methyl-1H-benzoimidazol-2yl}-butyric acid ethyl ester with thionyl chloride in dichloromethane at -1 - 22; for 17 h;
Stage #2: with hydrogenchloride in water at 75;
Steps:
9 Example 9: Synthesis of 4-[5-[bis(2-chloroethyl)amino]-1-methyl-1H-benzimidazol-2-yl]butanoic acid (9, Bendamustine hydrochloride hydrate)
Example 9
Synthesis of 4-[5-[bis(2-chloroethyl)amino]-1-methyl-1H-benzimidazol-2-yl]butanoic acid (9, Bendamustine hydrochloride hydrate)
250 g (0.7154 mol) compound (7) was dissolved in 2000 ml methylene chloride and 212 g (1.78 mol) thionyl chloride added at -1° C. within a period of 30 minutes.
Thereby the temperature rose briefly to ca. 4° C.
Following addition the reaction solution was stirred for a further 30 minutes at -1° C.
The solution was then stirred for ca. 16 h at ca. 22° C.
Thereafter the solvent and excess thionyl chloride were removed by distillation under vacuum.
To hydrolyse the ester the remaining residue (compound 8 as its hydrochloride) was treated with 2.6 kg 37% hydrochloric acid and 1.4 l water, heated to ca.
75° C. and held at this temperature for 30-40 minutes. 25 g activated carbon was then added and stirred for 10 minutes at 75° C.
The solution was filtered and concentrated under vacuum.
To crystallise crude compound (9) the residue was dissolved in 1000 ml water at ca.
55° C., the solution cooled to ca.
-2° C. and then held at this temperature for ca. 30 minutes.
The crude product (9) was filtered off, washed with 250 g water and 200 g acetone and dried for 2 h at ca. 35° C. under vacuum.
The yield of crude compound (9) was 245 g (0.5936 mol) and had a water content of 4.5% (83% of theory). 245 g compound (9) were dissolved in 330 g 37% hydrochloric acid at ca. 40° C., treated with 1.28 kg water (temperature ca. 35° C.) and 650 g acetone (temperature ca. 35° C.) and stirred for 10 minutes. Crystallisation was initiated by the addition of 0.5 g Bendamustine hydrochloride hydrate, the mixture cooled within a period of 2 h to ca. -20° C. and then held at this temperature for ca. 90 minutes. The precipitate was filtered under suction. The crystals were washed initially with a mixture of 120 g water and 90 g acetone and subsequently with 275 g acetone. The pure Bendamustine hydrochloride hydrate was dried for ca. 2 h at ca. 35° C. under vacuum. Thus 225 g (0.545 mol) of pure (>99.8% content) compound (9) was obtained with an overall yield of 76.2% for this step. Through drying of the Bendamustine hydrochloride hydrate under vacuum at ca. 50° C. the water content could be adjusted to ca. 1% in contrast to 4.4% of the mono hydrates.
References:
Heyl Chemisch-pharmazeutische Fabrik GmbH & Co. KG;Frey, Michael;Walther, Dirk-Detlef US2014/31560, 2014, A1 Location in patent:Paragraph 0113; 0114; 0115; 0116; 0117; 0118
![5-[Bis(2-chloroethyl)aMino]-1-Methyl-1H-benziMidazole-2-butanoic Acid Methyl Ester](/CAS/GIF/109882-25-9.gif)
109882-25-9
24 suppliers
$504.33/5MG

3543-75-7
376 suppliers
$35.00/10 mg
![1H-Benzimidazole-2-butanoic acid, 5-[bis(2-hydroxyethyl)amino]-1-methyl-, 1-methylethyl ester](/CAS/20180808/GIF/1313020-24-4.gif)
1313020-24-4
1 suppliers
inquiry

3543-75-7
376 suppliers
$35.00/10 mg
![5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid methyl ester](/CAS/GIF/109882-31-7.gif)
109882-31-7
14 suppliers
inquiry

3543-75-7
376 suppliers
$35.00/10 mg
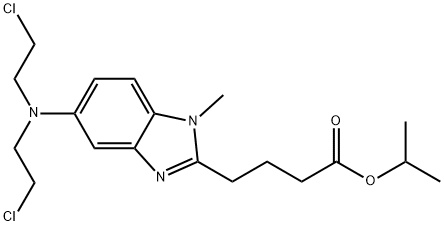
1313020-25-5
40 suppliers
$250.00/1mg

3543-75-7
376 suppliers
$35.00/10 mg