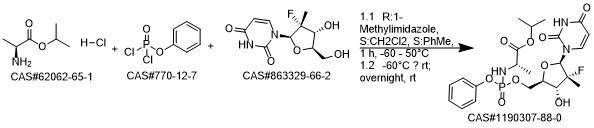
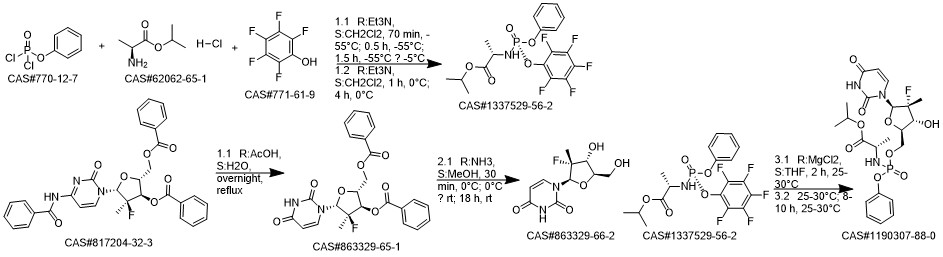
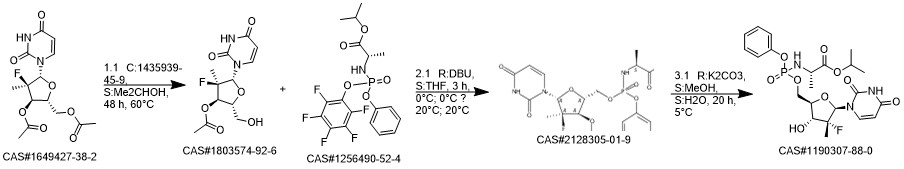
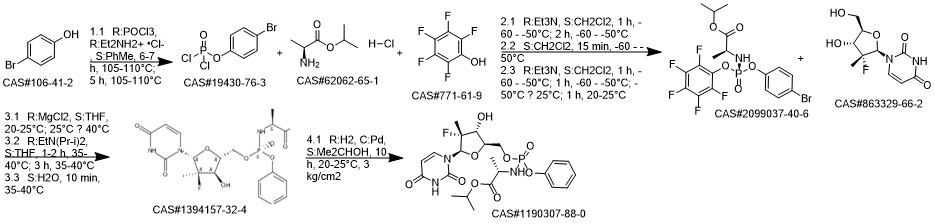
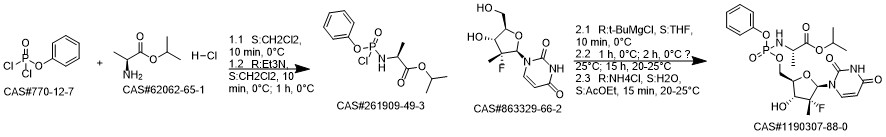
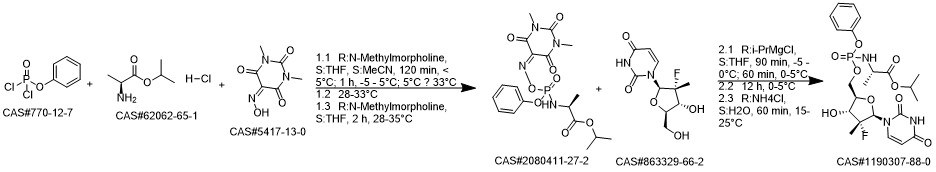
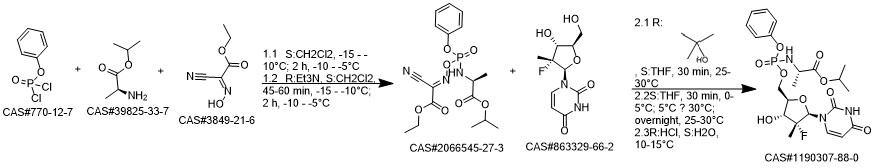
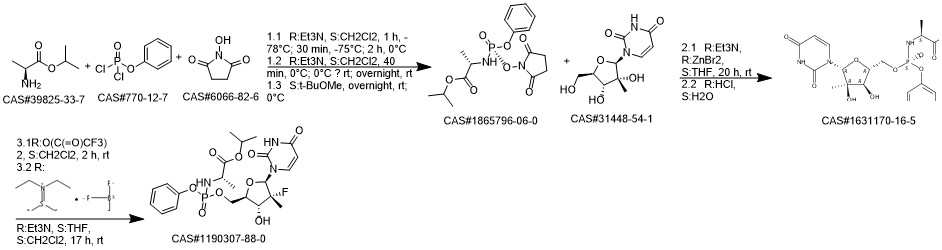
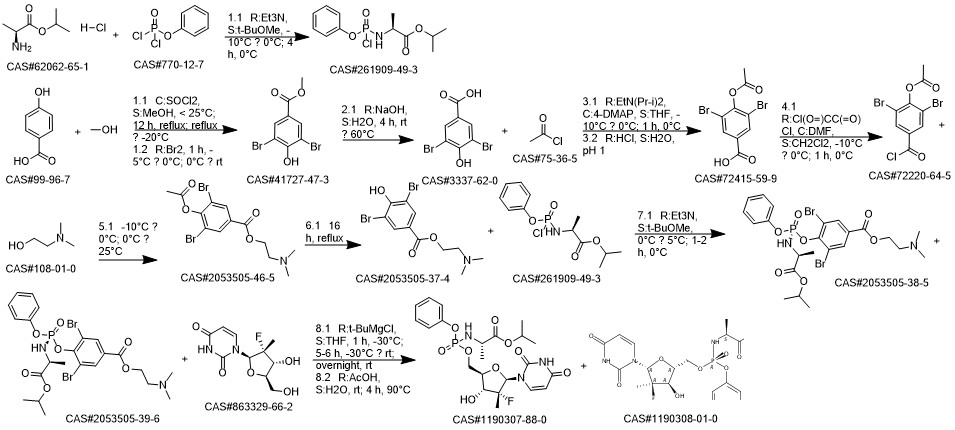
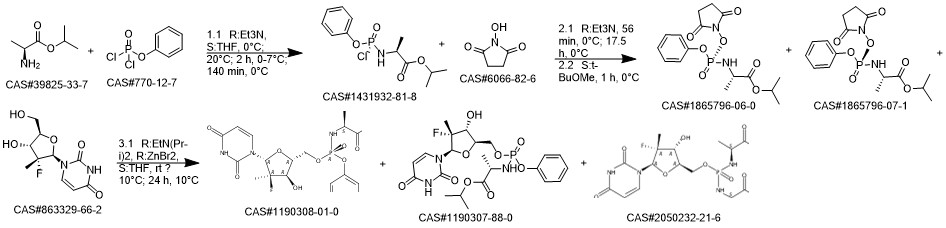
Sofosbuvir synthesis
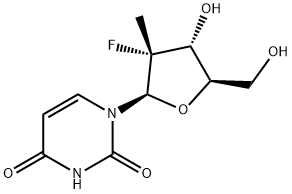
- Product Name:Sofosbuvir
- CAS Number:1190307-88-0
- Molecular formula:C22H29FN3O9P
- Molecular Weight:529.45
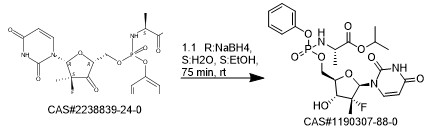
Reference: Wilhelm, Thorsten; Schoene, Olga. Synthesis of sofosbuvir nucleoside phosphoramidate via hydride reduction of ketone. WO 2018134343. (Sandoz AG, Switz)
863329-66-2
414 suppliers
$5.00/50mg
![N-[(S)-(2,3,4,5,6-pentafluorophenoxy)phenoxyphosphinyl]-L-alanine 1-Methylethyl ester](/CAS/GIF/1334513-02-8.gif)
1334513-02-8
309 suppliers
$12.00/5g

1190307-88-0
553 suppliers
$50.00/5mg
Yield:1190307-88-0 97.5%
Reaction Conditions:
with pyridine;aluminum (III) chloride in dichloromethane at 20;Reagent/catalyst;Temperature;
Steps:
1-8 Example 1
Add 20.0 g (0.0768 mol) of Compound 1 to the reaction flask, add 40.0 g (0.0882 mol) of Compound 2 and 150 g of dichloromethane, cool to 0 ° C, add aluminum trichloride 3.1 g (0.0232 mol), and add pyridine dropwise 24.3g (0.307mol); then the reaction was performed at 20 ° C, and the reaction was stopped when the content of sofosbuvir was no longer increased by HPLC. At this time, the HPLC data related to compound 1 in the reaction solution:To the reaction solution, 120 g of 10% dilute hydrochloric acid was added to quench the reaction, 300 g of ethyl acetate was extracted, the solvent was recovered under reduced pressure, and 39.68 g of pure Sofibuvir was obtained by purification, with a yield of 97.5%.
References:
CN110950919,2020,A Location in patent:Paragraph 0030-0067

863329-66-2
414 suppliers
$5.00/50mg
![N-[(S)-(4-Nitrophenoxy)phenoxyphosphinyl]-L-alanine 1-Methylethyl ester](/CAS/GIF/1256490-31-9.gif)
1256490-31-9
57 suppliers
inquiry

1190307-88-0
553 suppliers
$50.00/5mg

863329-66-2
414 suppliers
$5.00/50mg
![L-Alanine, N-[(2,3,4,5,6-pentafluorophenoxy)phenoxyphosphinyl]-, 1-Methylethyl ester](/CAS/20150408/GIF/1256490-52-4.gif)
1256490-52-4
57 suppliers
$31.00/1g

1190307-88-0
553 suppliers
$50.00/5mg

863329-66-2
414 suppliers
$5.00/50mg
![L-Alanine, N-[(R)-(2,3,4,5,6-pentafluorophenoxy)phenoxyphosphinyl]-, 1-Methylethyl ester](/CAS/20150408/GIF/1337529-56-2.gif)
1337529-56-2
43 suppliers
inquiry

1190307-88-0
553 suppliers
$50.00/5mg
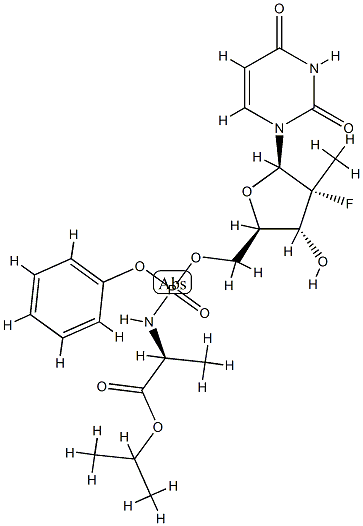
1064684-44-1
19 suppliers
inquiry

1190307-88-0
553 suppliers
$50.00/5mg