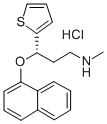
Duloxetine hydrochloride synthesis
- Product Name:Duloxetine hydrochloride
- CAS Number:136434-34-9
- Molecular formula:C18H20ClNOS
- Molecular Weight:333.88

321-38-0
580 suppliers
$6.00/10g

116539-55-0
304 suppliers
$8.00/1g

136434-34-9
631 suppliers
$25.00/10mg
Yield:136434-34-9 93%
Reaction Conditions:
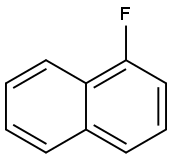
Stage #1:1-Fluoronaphthalene;(S)-3-methylamino-1-(2-thienyl)-1-propanol with sodium hydride in paraffin oil (nujol);dimethyl sulfoxide at 20 - 50;
Stage #2: with hydrogenchloride;acetic acid in Isopropyl acetate at 0 - 15; for 5 h;
Steps:
1
NaH (60 % in paraffin oil, 2.92 g) was suspended in DMSO (75 mL). After mixing of hydride (about 10 minutes) (S)-3-methyl-amino- 1 -(2-thienyl)- 1 -propanol (l Og) at 20 °C was added carefully. Stirring was started slowly and the reaction mixture was heated to 30-40 °C. A solution of F-naphthalene (9 mL) in DMSO (10 mL) was then added dropwise in about 30 minutes. Red-brownish suspension was heated to 40-50 °C for 9 to 18 hours. Heating was stopped, the mixture was cooled to 23 °C and quenched with a slow dropwise addition into ice-cold solution of water (200 mL) and acetic acid (12 mL). It was heated to 20 °C (pH ~ 5-5.5) and hexane (80 mL) was added. After extraction the phases were separated and the aqueous phase was alkalized with 50 % NaOH (12 mL). After complete addition of hydroxide iPrOAc (lOOmL) was added to the aqueous phase and during vigorous stirring the two-phase system was heated to 20 °C. The phases were separated and the aqueous phase was extracted one more time with iPrOAc (100 mL). The combined organic phases were rinsed 2X with water (2 X 80 mL) and treated for 30 minutes in hot with activated carbon. It was hot filtered and the filtrate was cooled. Into the cooled extract acetic acid (5 mL, 15+/-5°C) was added and the product was precipitated slowly with 2.5M HC1 (23 mL) in iPrOAc under vigorous stirring. The white suspension was stirred for 5 hours, cooled to 0-5 °C. It was filtered off and the filtrate was rinsed with iPrOAc. The product was dried in a vacuum drier for 5 hours. Obtained were 16.33 g (84 %) of crude duloxetine chloride (HPLC purity 99.87 %; 99.0 % ee), which was recrystallized from iPrOH. 15.18 g (93 %) of pure product (HPLC purity 99.94 % and 99.9 % ee) were obtained
References:
KRKA, D.D., NOVO MESTO;BOMBEK, Sergeja;MERSLAVIC, Marjo;BENKIC, Primoz;ZAJC, Natalija;VRECER, Franc WO2011/128370, 2011, A1 Location in patent:Page/Page column 21
132335-46-7
101 suppliers
inquiry

136434-34-9
631 suppliers
$25.00/10mg

321-38-0
580 suppliers
$6.00/10g

132335-44-5
333 suppliers
$6.00/1g

136434-34-9
631 suppliers
$25.00/10mg
![Carbamic acid, N-methyl-N-[(3S)-3-(1-naphthalenyloxy)-3-(2-thienyl)propyl]-, ethyl ester](/CAS/20210305/GIF/896446-94-9.gif)
896446-94-9
0 suppliers
inquiry

136434-34-9
631 suppliers
$25.00/10mg
947686-09-1
40 suppliers
inquiry

136434-34-9
631 suppliers
$25.00/10mg