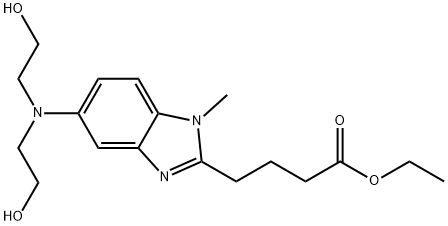
5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid ethyl ester synthesis
- Product Name:5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid ethyl ester
- CAS Number:3543-74-6
- Molecular formula:C18H27N3O4
- Molecular Weight:349.42
![5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid ethyl ester 5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid ethyl ester](/NewsImg/2024-03-27/6384713186339636192125883.png)
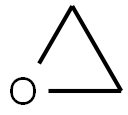
75-21-8
225 suppliers
$33.29/crm44609
3543-73-5
196 suppliers
$7.00/250mg
![5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid ethyl ester](/CAS/GIF/3543-74-6.gif)
3543-74-6
190 suppliers
$25.00/250mg
Yield:3543-74-6 78.53%
Reaction Conditions:
Stage #1:oxirane;ethyl 4‐(5‐amino‐1‐methyl‐1H‐benzo[d]imidazol‐2‐yl)butanoate with sodium acetate trihydrate in water;acetic acid at 0 - 25; for 23 h;
Stage #2: with potassium carbonate in dichloromethane at 20 - 25;
Steps:
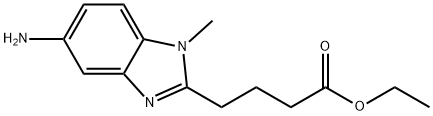
5 Preparation of Ethyl 4-{5-[bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazol-2-yl}butanoate (III)
Example-5
Preparation of Ethyl 4-{5-[bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazol-2-yl}butanoate (III)
Ethyl 4-[5-amino-1-methyl-1H-benzimidazol-2-yl)butanoate (II, 200.0 g, 0.763 mol) was added to DM Water (1.1 L).
Aqueous sodium acetate.3H2O (20.0 g sodium acetate.3H2O in 100 mL DM water) and acetic acid (400 mL) was added and agitated till complete dissolution of compound of the formula II.
The reaction mixture was cooled to 0-5° C. and ethylene oxide (270.0 g, 6.12 mole) was added maintaining the temperature of the reaction mixture at 0-5° C.
The reaction mixture was stirred at 0-5° C. for 5 hours.
The temperature of reaction mixture was raised to 20-25° C. and agitated at 20-25° C. for 18 hours.
After completion of the reaction, dichloromethane (2.0 L) was added at 20-25° C. followed by addition of aqueous solution of potassium carbonate (440.0 g potassium carbonate in 1.1 L DM water) portion wise at 20-25° C. to control the evaluation of effervescence and agitated at 20-25° C. for 5-10 minutes.
The layers were separated.
The organic layer (dichloromethane) was washed with DM water (1.0 L) twice and organic layer was concentrated under vacuum at 40-50° C. till viscous mass is obtained.
The viscous mass was dissolved in acetone (1.0 L), cooled to 0-5° C. and agitated at 0-5° C. for 1 hour.
The solid separated out was filtered, washed with chilled (0-5° C.) acetone (200.0 mL) and dried at 40-50° C. under vacuum for 6 hours to give the title compound (III, 210.0 g; 78.53%), with a purity of 99.06%.
References:
FRESENIUS KABI ONCOLOGY LIMITED;MISHRA, Bhuwan Bhaskar;KACHHADIA, Nikunj Shambhubhai;TOMAR, Vinod Singh;LAHIRI, Saswata US2014/121383, 2014, A1 Location in patent:Paragraph 0094

3543-73-5
196 suppliers
$7.00/250mg

540-51-2
237 suppliers
$17.00/5g
![5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid ethyl ester](/CAS/GIF/3543-74-6.gif)
3543-74-6
190 suppliers
$25.00/250mg
157-18-6
3 suppliers
inquiry

3543-73-5
196 suppliers
$7.00/250mg
![5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid ethyl ester](/CAS/GIF/3543-74-6.gif)
3543-74-6
190 suppliers
$25.00/250mg

64-17-5
715 suppliers
$10.00/50g
![1H-Benzimidazole-2-butanoic acid, 5-[bis(2-hydroxyethyl)amino]-2,3-dihydro-2-hydroxy-1-methyl-](/CAS/20210305/GIF/1464999-05-0.gif)
1464999-05-0
0 suppliers
inquiry
![5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid ethyl ester](/CAS/GIF/3543-74-6.gif)
3543-74-6
190 suppliers
$25.00/250mg

75-21-8
225 suppliers
$33.29/crm44609
![4-(5-amino-1-methyl-1H-benzo[d]imidazol-2-yl)butanoic acid](/CAS/20180703/GIF/914626-62-3.gif)
914626-62-3
12 suppliers
inquiry
![5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid ethyl ester](/CAS/GIF/3543-74-6.gif)
3543-74-6
190 suppliers
$25.00/250mg