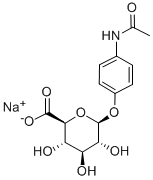
ACETAMINOPHEN GLUCURONIDE SODIUM SALT synthesis
- Product Name:ACETAMINOPHEN GLUCURONIDE SODIUM SALT
- CAS Number:120595-80-4
- Molecular formula:C14H16NNaO8
- Molecular Weight:349.27
30824-21-6
23 suppliers
$62.50/50 mg

120595-80-4
75 suppliers
$35.00/5mg
Yield: 85%
Reaction Conditions:
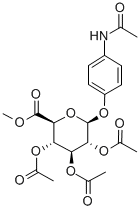
Stage #1:methyl <4-(acetamido)phenyl 2,3,4-tri-O-acetyl-β-D-glucopyranosid>uronate with water in tetrahydrofuran;methanol at 0;Inert atmosphere;
Stage #2: with potassium carbonate in tetrahydrofuran;methanol at 40; for 5 h;Inert atmosphere;Further stages;
Steps:
1.2.5. 4-Acetomidophenyl-β-d-glucopyranosiduronic acid (5) sodium salt
Methyl (4-acetamidophenyl 2,3,4-tri-O-acetyl-β-d-glucopyranosid)uronate (4) (1.00 g, 2.15 mmol) was dissolved in a solution of MeOH-THF-H2O (5:5:1, 40-50 mL) and the solution stirred under an N2 atmosphere at 0 °C. K2CO3 (0.6 g, 4.3 mmol) was added and the reaction mixture was heated to 40 °C for 5 h. The reaction mixture was cooled to room temperature and neutralised with strongly acidic ion-exchange resin (Amberlyst 15 H+ Form). The exchange resin was removed and the solution was clarified with charcoal. After removal of the charcoal on a bed of Celite the solvent was removed and the residue was dissolved in MeOH (50 mL). aq NaHCO3 solution (20 ml) was added and the solution heated to boiling. After heating for 10 min the solution was allowed to cool to room temperature and left to stir for 1 h. The resulting solid was isolated and recrystallised from aq EtOH to give the product as a white crystalline solid 0.63 g (85% yield). Mp 220-230 °C, (decomp.). IR (KBr) ν 3298 (O-H), 2935 (C-H), 1578 (CO), 1510 (CC), 1414 (CC), 1043 (C-O) cm-1; 1H NMR (D2O) δ 7.36 (d, 2H, J 9.2 Hz, Ar-H), 7.14 (d, 2H, J 9.2 Hz, Ar-H), 5.10 (d, 1H, J 6.8 Hz, H-1), 3.89 (d, 1H, J 9.6 Hz, H-5), 3.64-3.55 (m, 3H, H-2, 3, 4), 2.16 (s, 3H, NAc).
References:
Hayes, John A.;Eccles, Kevin S.;Lawrence, Simon E.;Moynihan, Humphrey A. [Carbohydrate Research,2012,vol. 349,p. 108 - 112] Location in patent:experimental part

92420-89-8
122 suppliers
$12.00/100mg

120595-80-4
75 suppliers
$35.00/5mg