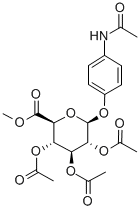
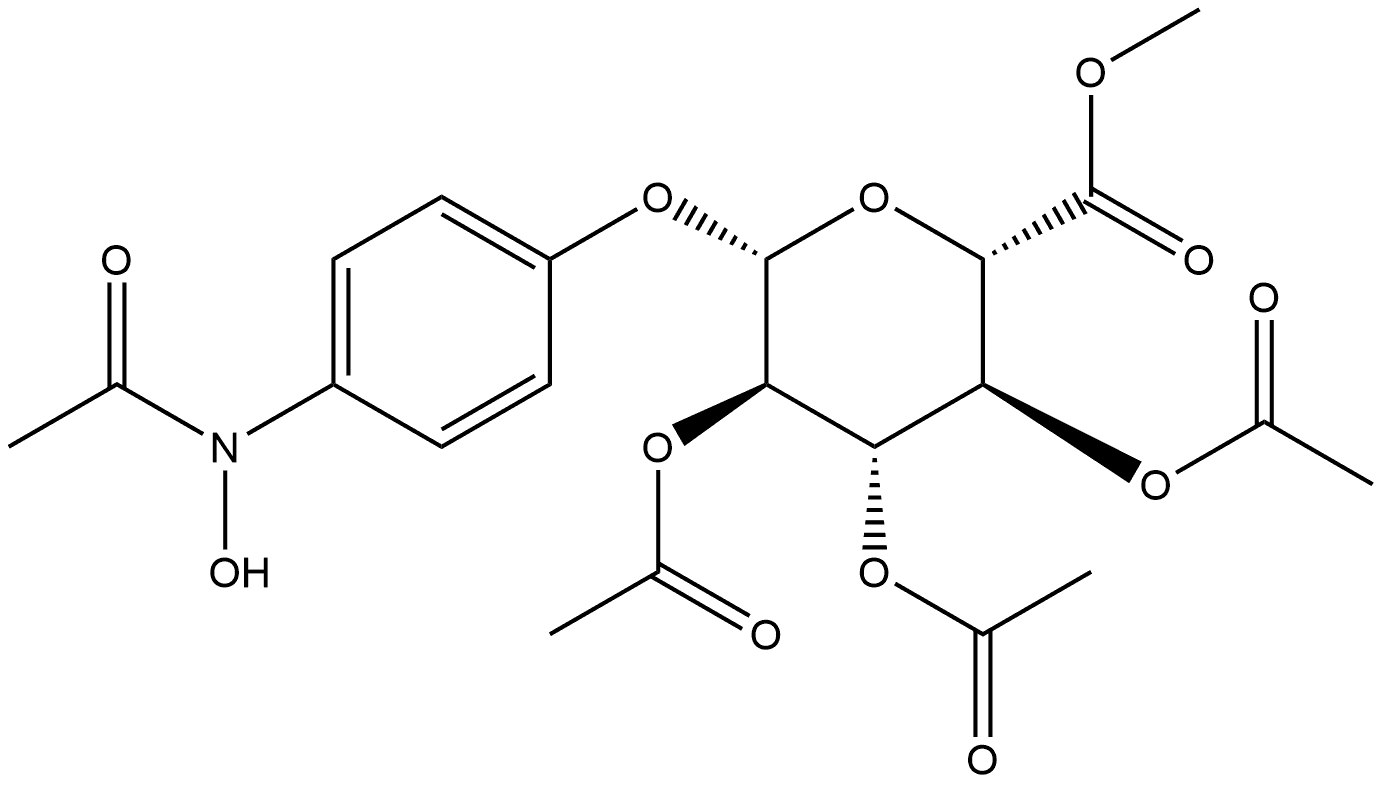
4-ACETAMIDOOPHENYL TRIACETYL-BETA-D-GLUCOPYRANOSIDURONIC ACID, METHYL ESTER synthesis
- Product Name:4-ACETAMIDOOPHENYL TRIACETYL-BETA-D-GLUCOPYRANOSIDURONIC ACID, METHYL ESTER
- CAS Number:30824-21-6
- Molecular formula:C21H25NO11
- Molecular Weight:467.42
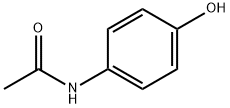
103-90-2
1052 suppliers
$9.00/1g

92420-89-8
122 suppliers
$12.00/100mg

30824-21-6
23 suppliers
$62.50/50mg
Yield:30824-21-6 76%
Reaction Conditions:
Stage #1: 4-acetaminophenol;methyl 2,3,4-tri-O-acetyl-1-O-(trichloroacetimidoyl)-α-D-glucopyranuronate in dichloromethane; for 0.5 h;Molecular sieve;
Stage #2: with boron trifluoride diethyl etherate in dichloromethane at 0;
Steps:
1.2.3. Methyl (4-acetamidophenyl-2,3,4-tri-O-acetyl-β-d-glucopyranosid)uronate (4)
Methyl 2,3,4-triacetyl-1-O-(trichloroacetimidoyl)-α-d-glucopyranouronate (3) (10.0 g, 21.0 mmol), paracetamol (3.5 g, 23 mmol) and 4 ? molecular sieves were stirred in dry CH2Cl2 for 30 min. BF3·Et2O (4 mL, 23 mmol) was added dropwise at 0 °C and the solution was allowed to stir overnight. The solvent volume was reduced, washed with satd aq Na2CO3 (2 × 30 ml), water (2 × 30 ml) dried with MgSO4 and concentrated in vacuo to yield a pale white solid. Column chromatography (40:1 CHCl3-MeOH) followed by recrystallisation from IPA gave a white crystalline solid (7.5 g, 76%). mp 214-216 °C, lit.23 213.5-214.5 °C; IR (KBr) ν 3300 (N-H), 3146-2969 (C-H), 1758 (CO), 1509 (Ar-H) cm-1. m/z (ESI): 468 (M+, 100%) 1H NMR (CDCl3) δ 7.42 (d, 2H, J 9 Hz, Ar-H), 7.2 (br s, 1H, NH), 6.96 (d, 2H, J 9 Hz, Ar-H), 5.35-5.23 (m, 3H, H-2, 3, 4), 5.07 (d, 1H, J 7.2 Hz, H-1), 4.14 (d, 1H, J 9.6 Hz, H-5), 3.74 (s, 3H, CO2Me), 2.16 (s, 3H, NAc), 2.06 (s, 3H, OAc), 2.05 (s, 3H, OAc), 2.04 (s, 3H, OAc). 13C NMR (CDCl3) δ 170.08 (CO), 169.35 (CO), 169.25 (CO), 168.29 (CO), 166.91 (CO), 153.28 (Cq, Ar) 133.28 (Cq, Ar), 121.66 (C-H, Ar), 117.82 (C-H, Ar), 99.64 (C1-H), 72.62 (C-H), 71.91 (C-H), 71.1 (C-H), 69.16 (C-H), 52.97 (CO2Me), 24.37 (NHAc), 20.75, 20.6, 20.49 (3 × OAc).
References:
Hayes, John A.;Eccles, Kevin S.;Lawrence, Simon E.;Moynihan, Humphrey A. [Carbohydrate Research,2012,vol. 349,p. 108 - 112] Location in patent:experimental part
463-51-4
14 suppliers
inquiry
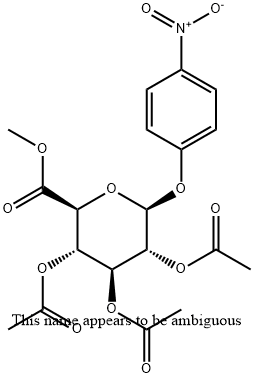
18472-49-6
43 suppliers
inquiry
78180-85-5
0 suppliers
inquiry

30824-21-6
23 suppliers
$62.50/50mg
21080-66-0
21 suppliers
$45.00/2.5mg

30824-21-6
23 suppliers
$62.50/50mg
108-24-7
5 suppliers
$14.00/250ML
25218-22-8
15 suppliers
$89.50/50mg

30824-21-6
23 suppliers
$62.50/50mg