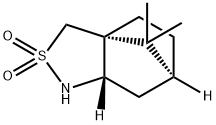
(2S)-Bornane-10,2-sultam synthesis
- Product Name:(2S)-Bornane-10,2-sultam
- CAS Number:108448-77-7
- Molecular formula:C10H17NO2S
- Molecular Weight:215.31
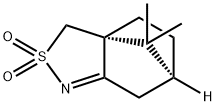
107869-45-4
84 suppliers
$13.00/1g

108448-77-7
255 suppliers
$6.00/1g
Yield:108448-77-7 97%
Reaction Conditions:
with hydrogen;Raney Nickel in ethanol; under 2068.65 Torr; for 2 h;
Steps:
1
EXAMPLE 1; (1R)-(+)-2,10-Camphor Sultam (Scheme 3, 3-1); To a solution of (-) camphor sulfonic acid (1.45 kg, 6.25 mol) in chloroform (7 L) under reflux condition is added thionyl chloride (0.896 kg, 7.5 mol) over a period of 1 h. The reaction mixture is refluxed for 16 h and then cooled to 4° C. using an ice bath. This cooled reaction mixture is added slowly to conc. NH4OH (15 L) while maintaining the temperature below 15° C. during the addition. After addition, the mixture is stirred for 4 h at room temperature. The organic layer is separated and the aqueous layer is extracted with chloroform (2×2 L). The combined organic extracts are washed with brine (4 L) and dried over MgSO4. After filtration, the filtrate is concentrated under vacuum and dried to give 1.2 kg (83%) of camphor sulfonamide. In one experiment at 145 g scale of camphor sulfonic acid, the use of dichloromethane instead of chloroform for the reaction and extraction gives comparable yields. To a suspension of the camphor sulfonamide (1.2 kg, 5.2 mol) in toluene (15 L) is added Amberlyst H+ resin (150 g), and the mixture is heated at reflux for 4 h with the water formed removed azeotropically using a Dean-Stark water separator. The reaction mixture is filtered hot to remove the resin. The filtrate on cooling gave a white solid which is collected by filtration to give 1.02 kg (92%) of the desired imine. To the above imine (100 g, 0.47 mol) in ethanol (0.75 L) is added Raney Nickel (100 g) and the mixture is hydrogenated at 40 psi for 2 h. The catalyst is removed by filtration and the filtrate concentrated under vacuum to give the product 3-1 as a white solid
References:
US2006/128789,2006,A1 Location in patent:Page/Page column 7
![3-Pyrrolidinecarboxylic acid, 2-(2-phenylethyl)-5-[[(3aR,6S,7aS)-tetrahydro-8,8-dimethyl-2,2-dioxido-3H-3a,6-methano-2,1-benzisothiazol-1(4H)-yl]carbonyl]-, methyl ester, (2R,3R,5R)-](/CAS/20210305/GIF/909191-06-6.gif)
909191-06-6
0 suppliers
inquiry

108448-77-7
255 suppliers
$6.00/1g
![3,4-Pyrrolidinedicarboxylic acid, 2-(2-phenylethyl)-5-[[(3aR,6S,7aS)-tetrahydro-8,8-dimethyl-2,2-dioxido-3H-3a,6-methano-2,1-benzisothiazol-1(4H)-yl]carbonyl]-, 3,4-dimethyl ester, (2R,3R,4S,5R)-](/CAS/20210305/GIF/909191-03-3.gif)
909191-03-3
0 suppliers
inquiry

108448-77-7
255 suppliers
$6.00/1g
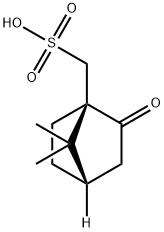
35963-20-3
483 suppliers
$6.00/25g

108448-77-7
255 suppliers
$6.00/1g
![4,7-Methano-1H-inden-1-one, 2,3,3a,4,7,7a-hexahydro-3-[[(3aR,6S,7aS)-tetrahydro-8,8-dimethyl-2,2-dioxido-3H-3a,6-methano-2,1-benzisothiazol-1(4H)-yl]carbonyl]-, (3R,3aS,4S,7R,7aR)-](/CAS/20210305/GIF/196303-41-0.gif)
