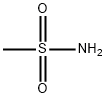
Methanesulfonamide synthesis
- Product Name:Methanesulfonamide
- CAS Number:3144-09-0
- Molecular formula:CH5NO2S
- Molecular Weight:95.12
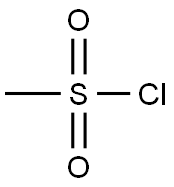
124-63-0
6 suppliers
$10.00/5g

3144-09-0
374 suppliers
$6.00/5g
Yield:3144-09-0 99%
Reaction Conditions:
with triethylamine in dichloromethane;ethyl acetate;
Steps:
7.A Preparation of 4-[(3,5-dimethyl-1-piperidinyl)sulfonyl]tetrahydro-N-hydroxy-2H-pyran-4-carboxamide
Part A: 3,5-Dimethylpiperadine (70% cis/30% trans, 7.0 mL, 53 mmol) and triethylamine (11.2 mL, 80 mmol) were slurried in CH2Cl2 (75 mL) and cooled to 0° C. A solution of methanesulfonyl chloride (6.2 mL, 80 mmol) in CH2Cl2 (25 mL) was slowly added, maintaining the temperature <10° C. with an ice bath. After the addition, the ice bath was removed and the reaction stirred 1 hr as it came to room temperature. After the disappearance of the starting material, the solvent was removed and the residue was taken up in ethyl acetate (200 mL) and H2O (50 mL). Once separated, the organic layer was washed with 5% KHSO4 (3*-50 mL) and brine (1*-50 mL). The organic layer was then dried over Na2SO4, filtered, and concentrated to afford the methyl sulfonamide an off white solid (10.0 g, 99% yield. 1H NMR showed the desired compound.
References:
US6372758,2002,B1
1516-70-7
26 suppliers
inquiry

3144-09-0
374 suppliers
$6.00/5g
362665-07-4
1 suppliers
inquiry
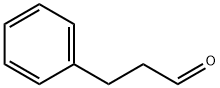
104-53-0
277 suppliers
$6.00/5g

3144-09-0
374 suppliers
$6.00/5g
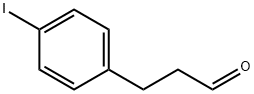
213264-51-8
3 suppliers
inquiry