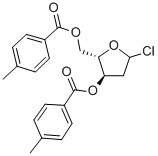
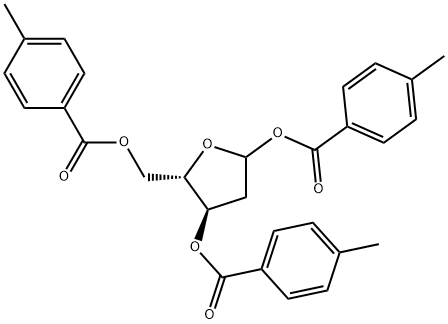
ALPHA-L-ERYTHRO-PENTOFURANOSYL CHLORIDE-2-DEOXY-BIS(4-METHYL BENZOATE) synthesis
- Product Name:ALPHA-L-ERYTHRO-PENTOFURANOSYL CHLORIDE-2-DEOXY-BIS(4-METHYL BENZOATE)
- CAS Number:141846-57-3
- Molecular formula:C21H21ClO5
- Molecular Weight:388.84
141846-58-4
0 suppliers
inquiry

141846-57-3
118 suppliers
$55.00/100mg
Yield:141846-57-3 90%
Reaction Conditions:
with hydrogenchloride;acetyl chloride in toluene at 5 - 15; for 1 h;
Steps:
6
EXAMPLE 6 Synthesis of 1-chloro-3o5-di-O-toluoyl-2-deoxy-L-ribofuranoside [Compound of formula (I) in which R is 4-methyl-benzoyl] To the toluenic solution of 1-0-methyl-3, 5-di-O-toluoyl-2-deoxy-L-ribofuranoside, prepared as described above in Example 4,77 ml (1. 08 mol) of acetyl chloride are added. The solution is cooled down to 5°C and hydrochloric acid is bubbled into the solution, controlling the bubbling so as to maintain the inner temperature below 15°C. After the addition the solution is left under stirring at 5°C, after about 30 minutes the product precipitates as white solid. After 1 hour a TLC check is performed in order to evaluate absence of the spot corresponding to the starting product. With a positive analytical check the operation is proceeded forward, otherwise the suspension is left under stirring for a further hour. The apparatus is put under vacuum for 2 hours to remove gaseous hydrochloric acid in excess, then the solid is filtrated and washed with 100 mi of toluene and with 50 ml of hexane. The solid is exsiccated under vacuum at 35-40°C until constant weight. 140 g (0.36 moles) of 1-chloro-3, 5-di-O-toluoyl-2-deoxy-L-ribofuranoside are obtained. Yield = 90%. 1H-NMR (CDCI3, 300 MHz): 5 ppm 8-7.89 (4H, dd, aromatic); 7. 28-7. 22 (4H, dd, aromatic); 6.48 (1H, d, H-1); 5.57 (1H, ddd, H-3); 4. 87 (1H, ddd, H-4); 4.64 (2H, AB system, CH2-5) ; 2. 81 (2H, m, CH2-2). 13C-NMR (CDCI3, 300 MHz): 5 ppm 166.63 and 166.39 (2 C=O) ; 144.51-126. 91 (C aromatic); 95.56 (C-1); 84.93 (C-4) ; 73.77 (C-3); 63.72 (C-5); 44. 75 (C-2); 21. 95 and 21.91 (2 CH3).
References:
WO2005/44832,2005,A1 Location in patent:Page/Page column 9-10
22837-36-1
5 suppliers
inquiry

141846-57-3
118 suppliers
$55.00/100mg
13039-64-0
5 suppliers
inquiry

141846-57-3
118 suppliers
$55.00/100mg
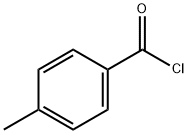
874-60-2
409 suppliers
$10.00/5g
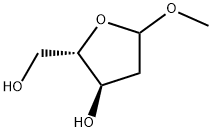
446251-73-6
42 suppliers
$100.00/500mg

141846-57-3
118 suppliers
$55.00/100mg
817621-14-0
0 suppliers
inquiry

141846-57-3
118 suppliers
$55.00/100mg