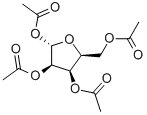
1,2,3,5-TETRA-O-ACETYL-BETA-L-RIBOFURANOSE synthesis
- Product Name:1,2,3,5-TETRA-O-ACETYL-BETA-L-RIBOFURANOSE
- CAS Number:144490-03-9
- Molecular formula:C13H18O9
- Molecular Weight:318.28
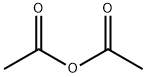
108-24-7
5 suppliers
$14.00/250ML
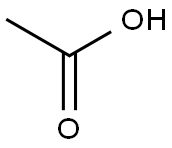
64-19-7
1553 suppliers
$10.00/25ML
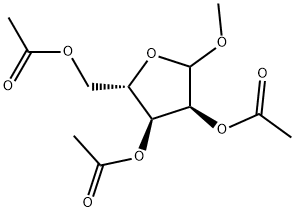
659722-56-2
1 suppliers
inquiry
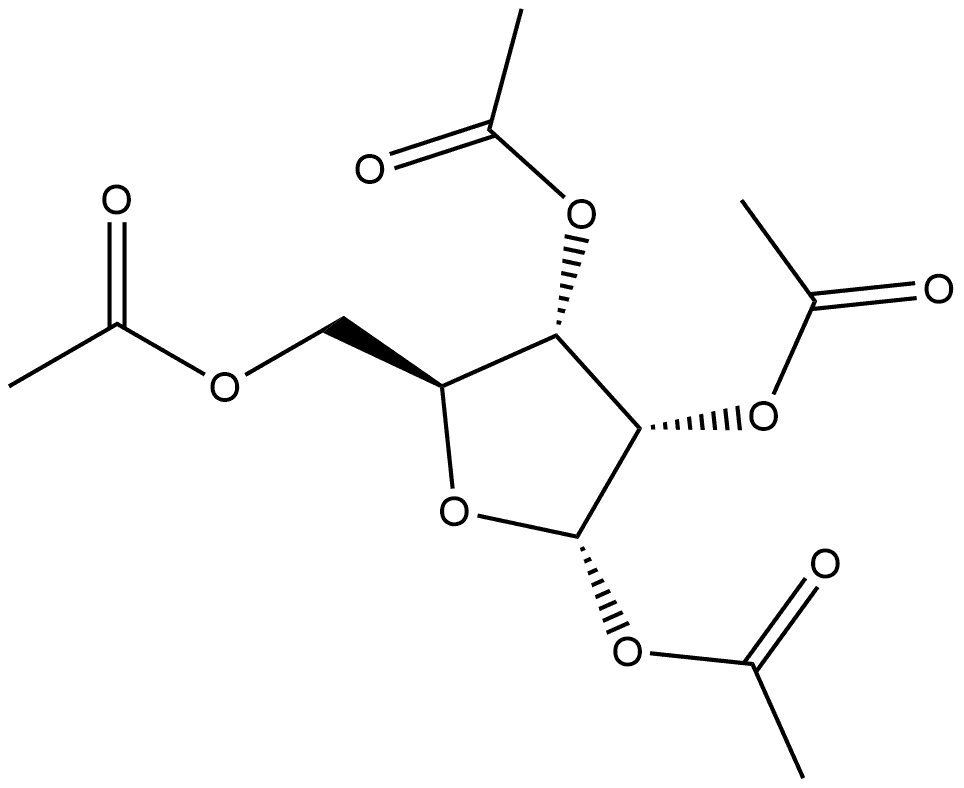
659722-55-1
1 suppliers
inquiry

144490-03-9
133 suppliers
$30.00/1g
Yield:88 % de
Reaction Conditions:
with pyridine;sulfuric acid in di-isopropyl ether at 5;Product distribution / selectivity;Inert atmosphere;
Steps:
B3
A 100-ml four-necked flask was subjected to nitrogen substitution. 2.97 g (purity: 98 wt%; corresponding to 10 mmol of L-ribose) of the 2,3,5-tri-O-acetyl-1-O-methyl-L-ribofuranose obtained at a yield of 93.5% from L-ribose was added to the flask by the same method as that of Example B1(1). Thereafter, 1.85 ml (2.0 equivalents) of acetic anhydride, 1.14 ml (2.0 equivalents) of acetic acid, and 0.64 ml (0.8 equivalents) of pyridine were added thereto. While stirring in an ice bath, 2.2 g (2.2 equivalents) of concentrated sulfuric acid was added dropwise thereto at an internal temperature of 0+/-5°C. The temperature was increased to room temperature, and the reaction solution was stirred for 1.5 hours. Thereafter, the reaction solution was maintained at a temperature of 0+/-5°C again in an ice bath, and 10 ml of diisopropyl ether was added thereto, followed by stirring in an ice bath for 4 hours. Thereafter, the reaction product was maintained at 5°C or lower in a refrigerator overnight. While stirring in an ice bath, 3.60 g of sodium acetate was added to the reaction product, and the obtained mixture was then stirred in an ice bath for 30 minutes. 30 ml of ethyl acetate and a saturated sodium bicarbonate aqueous solution were added to the reaction solution at room temperature until the aqueous layer was neutralized, followed by liquid separation. The aqueous layer was extracted with 30 ml of ethyl acetate, and organic layers were then gathered. The gathered layers were washed with 20 ml of a saturated sodium bicarbonate aqueous solution and then with 20 ml of a saturated sodium chloride aqueous solution 2 times. The organic layer was dried over anhydrous sodium sulfate, and the drying agent was then removed by filtration, concentrated under reduced pressure. Thus, 3.67 g of crude 1,2,3,5-tetra-O-acetyl-L-ribofuranose was obtained in the form of yellow oil. The obtained compound was analyzed by HPLC. As a result, it was found that the crude product contained 2.63 g of 1,2,3,5-tetra-O-acetyl-L-ribofuranose, that the ratio of α-/β-anomers was 6/94, and that the total reaction yield of the β-anomer from L-ribose was 73%.
References:
EP2105445,2009,A1 Location in patent:Page/Page column 14

108-24-7
5 suppliers
$14.00/250ML

64-19-7
1553 suppliers
$10.00/25ML

659722-56-2
1 suppliers
inquiry

144490-03-9
133 suppliers
$30.00/1g
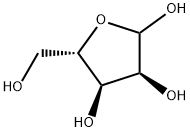
41546-21-8
11 suppliers
inquiry

108-24-7
5 suppliers
$14.00/250ML

144490-03-9
133 suppliers
$30.00/1g

108-24-7
5 suppliers
$14.00/250ML
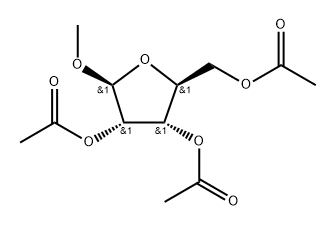
206269-25-2
3 suppliers
inquiry

659722-55-1
1 suppliers
inquiry

144490-03-9
133 suppliers
$30.00/1g

7664-93-9
6 suppliers
$23.30/1090731000

206269-25-2
3 suppliers
inquiry

144490-03-9
133 suppliers
$30.00/1g