
2-Deoxy-alpha-D-erythropentofuranosyl chloride 3,5-bis(4-methylbenzoate) synthesis
- Product Name:2-Deoxy-alpha-D-erythropentofuranosyl chloride 3,5-bis(4-methylbenzoate)
- CAS Number:4330-21-6
- Molecular formula:C21H21ClO5
- Molecular Weight:388.84
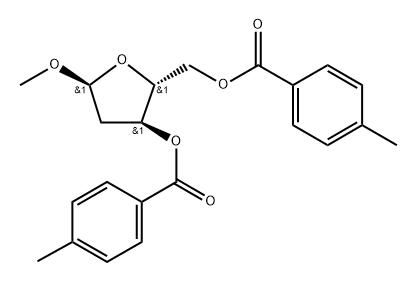
78185-64-5
14 suppliers
inquiry

4330-21-6
176 suppliers
$7.00/250mg
Yield:4330-21-6 58%
Reaction Conditions:
with hydrogenchloride;glacial acetic acid;acetyl chloride at 20; for 0.5 h;
Steps:
2.3 Synthesis of compound 4
To a solution of (2R,3S,5S)-5-methoxy-2-(((4- methylbenzoyl)oxy)methyl)tetrahydrofuran-3-yl 4-methylbenzoate (250 g, 0.65 mol, 1 eq) in acetic acid (393 mL) was added a freshly prepared solution of saturated HC1 in acetic acid (using acetyl chloride and acetic acid) (618 mL) slowly at room temperature. After completion of the addition, further acetyl chloride (50 mL) was added slowly at room temperature. The reaction mixture was stirred for a further 30 minutes, upon which time a colourless precipitate formed that was isolated by filtration to give (2R,3S,5R)-5-chloro-2- (((4-methylbenzoyl)oxy)methyl)tetrahydrofuran-3-yl 4-methylbenzoate as a colourless solid (145 g, 58 %). LCMS: 407 ( 8.10 - 7.84 (m, 5H), 7.34 - 7.20 (m, 5H), 6.52 (d, J = 5.1 Hz, 1H), 5.61 (ddd, J = 7.4, 2.8, 1.1 Hz, 1H), 4.91 (q, J = 3.5 Hz, 1H), 4.75 - 4.61 (m, 2H), 2.92 (ddd, J = 15.0, 7.5, 5.2 Hz, 1H), 2.79 (dd, J = 15.2, 1.1 Hz, 1H), 2.47 (d, J - 4.8 Hz, 6H).
References:
WO2022/81739,2022,A1 Location in patent:Page/Page column 32-33

4330-34-1
63 suppliers
$45.00/250mg

4330-21-6
176 suppliers
$7.00/250mg
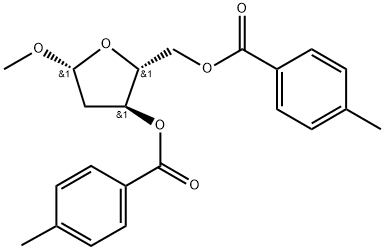
78185-65-6
10 suppliers
inquiry

4330-21-6
176 suppliers
$7.00/250mg
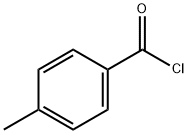
874-60-2
409 suppliers
$10.00/5g

4330-21-6
176 suppliers
$7.00/250mg
13222-85-0
57 suppliers
$21.00/1g

4330-21-6
176 suppliers
$7.00/250mg