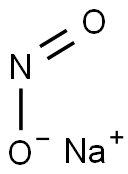??? ???
|
|
??? ??? ??
- ???
- 271 °C (lit.)
- ?? ?
- 320 °C
- ??
- 2.17g/cm3
- ?? ??
- 2-8°C
- ???
- ?? ?: ml? 1 - 2l ???? H2???
- ??? ??
- ??
- ??
- ?? ?? ??
- Specific Gravity
- 2.168
- ??
- ?? ??
- pH ??
- 9
- ??????(pH)
- 9 (100g/l, H2O, 20℃)
- ???
- 820g/L(20℃)
- ??
- Hygroscopic
- Merck
- 14,8648
- ???
- ????. ???, ????, ??? ? ?? ??? ??, ?? ?? ??? ???? ????. ??? ??? ???? ??? ??? ? ????. ???.
- InChIKey
- LPXPTNMVRIOKMN-UHFFFAOYSA-M
- CAS ??????
- 7632-00-0(CAS DataBase Reference)
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | O,T,N,Xn | ||
|---|---|---|---|
| ?? ???? ?? | 8-25-50-22-36/38 | ||
| ????? | 45-61-36-26 | ||
| ????(UN No.) | UN 3219 5.1/PG 3 | ||
| WGK ?? | 3 | ||
| RTECS ?? | RA1225000 | ||
| F ?????? | 10 | ||
| ?? ?? ?? | 914 °F | ||
| TSCA | Yes | ||
| ?? ?? | 5.1 | ||
| ???? | III | ||
| HS ?? | 28341000 | ||
| ?? ?? ??? | 7632-00-0(Hazardous Substances Data) | ||
| ?? | LD50 orally in rats: 180 mg/kg (Smyth) | ||
| ???? ?? | KE-31546 | ||
| ?????? ??? | 97-1-167 | ||
| ?? ? ???? | ????: ????; ???(??)????: ??? ?? ? ?? 25% ?? ??? ??? |
??? ??? C??? ??, ??, ??
??
???????, ?? ?? ??? ???? ??.?? ??
2NaOH + NO2 + NO → 2NaNO2 + H2ONO2 + NO? HNO2 ? ??? ? ??.
??
?? ?????? ?? ??? ???, ?? ?????? ??? ???, ????, ??? ??, ??? ???, ??? ???, ???? ?? ??? ??. ??????, ????(KNO3)? ?? ???, ? ?? ?? ???? ???? ?? ?.??
???? ??? ?? 4~6g ????[2], ?? ??? ???? ?????? ???? ?????? ???? ??? ????? ???????? ??, ??? ??? ??. ???? ?? ???? ??? ????? ??? ?? ??? ??? ??? ? ?? ???, ???? ???? ?? ????? ???? ?????? ?? ??? ???.??? ????? ??? ??? ??? ??? ??? ??.
????
? (1) ?? : ? ?? 1g? ? 20mL? ?? ?, ? ??? ?? ?? ????? ??.
??(2) ??? : ? ?? 1g? ?? ?? 500mL? ?? ? 10mL? ?? 3mL? ??? ??? ???? ??? ???? ?? ? ?? ???? 6mL? ??? ?? ?????? ?? ??????? ?? ??? ?, ? ?? 0.01N ?? 0.4mL? ???? ? ????? ??.
??(3) ??? : ? ?? 1g? ?? ?? 100mL? ?? ? ? 10mL? ?? 1mL? ??? ????? ?????? ? ???? ???? 1mL ? ? 20mL? ??? ?? ?? ?????? ?? ??????? ?? ??? ?, ? ?? 0.01N ?? 0.5mL? ???? ? ????? ??.
??(4) ?? : ? ??? ?????? ?? ??? ?, ? ?? 4.0ppm ????? ??.
??(5) ? : 「???????」? ???? (2)? ?? ????(2.0ppm ??).
??(6) ?? : ? ??? ?????? ?? ??? ?, ? ?? 1.0ppm ????? ??.
????
? ? ??? ????? ? ???? ? ????? ??? ????.
???
? ? ??? ??? ?? ? 1g? ??? ?? ?? ?? 100mL? ?? ? ? 10mL? ??? ?? 0.1N ???????? 50mL, ? 100mL ? ?? 5mL? ?? ?????? ?? ??? ?? ???? ??? 5?? ??? ?? 0.1N ???? 25mL? ??? ? 80℃? ???? ??? ? ??? ??? 0.1N ?????????? ????. ?? ?? ???? ???? ??.
0.1N ???????? 1mL = 3.450mg NaNO2
??
Sodium nitrite is similar in name and use to sodium nitrate. Both are preservatives used in processed meats, such as salami, hot dogs, and bacon. Sodium nitrite has been synthesized by several chemical reactions that involve the reduction of sodium nitrate. Industrial production of sodium nitrite is primarily by the absorption of nitrogen oxides into aqueous sodium carbonate or sodium hydroxide. Over the years, sodium nitrite has raised some concerns about its safety in foods, but it remains in use and there are indications that it may actually be healthy. Sodium nitrite was developed during the 1960s. In 1977, the US Department of Agriculture (USDA) considered banning it but the USDA’s final ruling on the additive came out in 1984, allowing its use. Studies in the 1990s indicated some adverse effects of sodium nitrite, for instance the potential to cause childhood leukemia and brain cancers. In the late 1990s, the National Toxicity Program (NTP) began a review of sodium nitrite and proposed listing sodium nitrite as a developmental and reproductive toxicant, but a report in 2000 by NTP proposed that sodium nitrite is not a toxic substance and removed it from the list of developmental and reproductive toxicants. It is now believed that it can help with organ transplants and leg vascular problems, while preventing heart attacks and sickle cell disease.??? ??
Sodium nitrite, NaN02, is a fire-hazardous, air-sensitive, yellowish white powder that is soluble in water and decomposes at temperatures above 320°C (608 °F). Sodium nitrite is used as an intermediate for dye stuffs and for pickling of meat, in dyeing of textiles, in rustproofing, in medicine, and as a reagent in organic chemistry.??
Sodium nitrite is used to fix the colors in preserved fish and meats. It is also important(along with sodium chloride) in controlling the bacterium Clostridium botulinum, which causes botulism. Lunch meats, hams, sausages, hot dogs, and bacon are usually preserved this way.In medicines, it is a vasodilator, intestinal relaxant, bronchodilator, and an antidote to cyanide and hydrogen sulfide poisoning.
Sodium nitrite is produced in the human body by the action of saliva on sodium nitrate, and is important in controlling bacteria in the stomach, to prevent gastroenteritis. The body produces more sodium nitrite than is consumed in food.
Sodium nitrite can react with proteins in the stomach or during cooking, especially in high heat (such as frying bacon), to form carcinogenic N-nitrosamines.To prevent this, ascorbic acid or erythorbic acid is commonly added to cured meats.
manufacture of diazo dyes, nitroso Compounds, and in many other processes of manufacture of organic chemicals; dyeing and printing textile fabrics; bleaching flax, silk, and linen.
?? ??
Sodium nitrite, yellowish-white solid, soluble, formed (1) by reaction of nitric oxide plus nitrogen dioxide and sodium carbonate or hydroxide, and then evaporating, (2) by heating sodium nitrate and lead to a high temperature, and then extracting the soluble portion (lead monoxide insoluble) with H2O and evaporating. Used as an important reagent (diazotizing) in organic chemistry.??? ?? ??
Soluble in water.?? ???
Sodium nitrite is an oxidizing agent. Mixtures with phosphorus, tin(II) chloride or other reducing agents may react explosively [Bretherick 1979 p. 108-109]. If contaminated by ammonium compounds, spontaneous decomposition can occur and resulting heat may ignite surrounding combustible material. Reacts with acids to form toxic nitrogen dioxide gas. Mixing with liquid ammonia forms dipotassium nitrite, which is very reactive and easily explosive [Mellor 2, Supp. 3:1566 1963]. Melting together wilh an ammonium salt leads to a violent explosion [Von Schwartz 1918 p. 299]. A mixture with potassium cyanide may cause an explosion. Noncombustible but accelerates the burning of all combustible material. If large quantities are involved in fire or if the combustible material is finely divided, an explosion may result. When a little ammonium sulfate is added to fused potassium nitrite, a vigorous reaction occurs attended by flame [Mellor 2:702. 1946-47].???
Dangerous fire and explosion risk when heated to 537C (1000F) or in contact with reducing materials; a strong oxidizing agent. Carcinogen in test animals; its use in curing fish and meat products is restricted to 100 ppm.????
Ingestion (or inhalation of excessive amounts of dust) causes rapid drop in blood pressure, persistent and throbbing headache, vertigo, palpitations, and visual disturbances; skin becomes flushed and sweaty, later cold and cyanotic; other symptoms include nausea, vomiting, diarrhea (sometimes), fainting, methemoglobinemia. Contact with eyes causes irritation.Safety Profile
Human poison by ingestion. Experimental poison by ingestion, inhalation, subcutaneous, intravenous, and intraperitoneal routes. Human systemic effects by ingestion: motor activity changes, coma, decreased blood pressure with possible pulse rate increase without fall in blood pressure, arteriolar or venous dlation, nausea or vomiting, and blood me themoglo binemiacarboxyhemoglobinemia. Experimental teratogenic and reproductive effects. An eye irritant. Questionable carcinogen with experimental neoplas tigenic and tumorigenic data. Human mutation data reported. It may react with organic amines in the body to form carcinogenic nitrosamines. Flammable; a strong oxidizing agent. In contact with organic matter, will ignite by friction. May explode when heated to over 100O0F or on contact with cyanides, NH4' salts, cellulose, LI, (K + NH3), Na2S203. Incompatible with aminoguanidine salts, butadene, phthalic acid, phthalic anhydride, reductants, sodlum amide, sodmm disulfite, sodium thocyanate, urea wood. When heated to decomposition it emits toxic fumes of NOx and NaaO. See also NITRITES.Purification Methods
Crystallise NaNO2 from hot water (0.7mL/g) by cooling to 0o, or from its own melt. Dry it over P2O5. (See KNO2.)??? ??? ?? ?? ? ???
???
?? ??
??? ??? ?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Shenyang Simchoice Chemical Co.,Ltd | +86-024-23769576 +86-15040101888 |
sales@simchoicechem.com | China | 254 | 58 |
| Hebei Weibang Biotechnology Co., Ltd | +8615531157085 |
abby@weibangbio.com | China | 8810 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12835 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5889 | 58 |
| Firsky International Trade (Wuhan) Co., Ltd | +8615387054039 |
admin@firsky-cn.com | China | 427 | 58 |
| Hebei Kingfiner Technology Development Co.Ltd | +86-15532196582 +86-15373005021 |
lisa@kingfinertech.com | China | 3010 | 58 |
| Shaanxi Haibo Biotechnology Co., Ltd | +undefined18602966907 |
qinhe02@xaltbio.com | China | 997 | 58 |
| Capot Chemical Co.,Ltd. | +86-(0)57185586718 +86-13336195806 |
sales@capot.com | China | 29791 | 60 |
| Hangzhou Bayee Chemical Co., Ltd. | 0086-571-86990109 |
rachelhoo@bayeechem.com | China | 104 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21634 | 55 |