39552-01-7
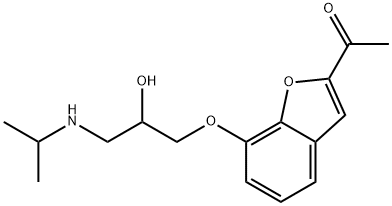 39552-01-7 結(jié)構(gòu)式
39552-01-7 結(jié)構(gòu)式
基本信息
中文別名
苯呋洛爾2-乙酰-7-(2-羥基-3-異丙胺丙氧)-苯并呋喃
英文別名
Befunolol2-acetyl-7-(2-hyroxy-3-isopropylaminopropoxy)benzofuran
2-Acetyl-7-(2-hydroxy-3-isopropylaminopropoxy)benzofuran
(-)-2-Acetyl-7-[(S)-2-hydroxy-3-isopropylaminopropoxy]benzofuran
(+)-2-Acetyl-7-[(R)-2-hydroxy-3-isopropylaminopropoxy]benzofuran
7-(2-hydroxy-3-(isopropylamino)propoxy)-2-benzofuranyl methyl ketone
ketone, 7-(2-hydroxy-3-(isopropylamino)propoxy)-2-benzofuranyl methyl
7-[2-Hydroxy-3-(iso-propylamino)propoxy]-2-benzofuranyl methyl ketone
1-(7-(2-hydroxy-3-((1-methylethyl)amino)propoxy)-2-benzofuranyl)ethanone
1-(7-(2-hydroxy-3-((1-methylethyl)amino)propoxy)-2-benzofuranyl)-ethanon
所屬類別
原料藥:抗心律失常藥物理化學(xué)性質(zhì)
外觀性狀結(jié)晶。熔點(diǎn)115℃。苯呋洛爾鹽酸鹽(C16H21NO4·HCI),熔點(diǎn)163℃。
熔點(diǎn)115°
沸點(diǎn)433.35°C (rough estimate)
密度1.1049 (rough estimate)
折射率1.5500 (estimate)
儲(chǔ)存條件Hygroscopic, -20°C Freezer, Under inert atmosphere
溶解度氯仿(微溶)、DMSO(微溶、加熱)
形態(tài)固體
顏色白色至灰白色
CAS 數(shù)據(jù)庫39552-01-7
制備方法
方法1
由2-乙酰-7-羥基苯并呋喃與3-氯-1,2-環(huán)氧丙烷反應(yīng)后再與異丙胺作用而得。取8.8g2-乙酰-7-羥基苯并呋喃中,加入80ml3-氯-1,2-環(huán)氧丙烷和0.2g鹽酸哌啶。將混合物加熱至105℃,反應(yīng)3h。反應(yīng)后蒸發(fā)出過量的3-氯-1,2-環(huán)氧丙烷。生成物在減壓下蒸餾,得9.3g2-乙酰-7-(2,3-環(huán)氧丙氧)苯并呋喃,沸點(diǎn)175-176℃(93Pa)。取6g上述生成物溶于30ml乙醇中,加10ml異丙胺,混合物回流40min,由反應(yīng)混合物中蒸除溶劑,得到的殘余物用環(huán)己烷-丙酮重結(jié)晶,得6g苯呋洛爾。常見問題列表
生物活性
(±)-Befunolol 是一種 β-腎上腺素能受體阻斷劑。靶點(diǎn)
Adrenoreceptor
體外研究
A β-adrenoceptor blocking agent, Befunolol, is found to has intrinsic sympathomimetic activities in isolated right atria, trachea and taenia caecum of guinea pig (intrinsic activities are 0.22-0.28). The pD2-values of Befunolol estimated in the isolated organs are significantly different from its pA2-values against isoprenaline. Befunolol interacts with the beta-adrenoceptor where there may be two different sites: one site for agonistic action and the other for competitive antagonistic action. The intrinsic activity of Befunolol may be equal to its selectivity for both the sites.