| Identification | More | [Name]
Diethyl oxalate | [CAS]
95-92-1 | [Synonyms]
AKOS BBS-00004457
DIETHYL ETHANEDIOATE
DIETHYL OXALATE
Ethanedioic acid diethyl ester
ETHYL OXALATE
OXALIC ACID DIETHYL ESTER
RARECHEM AL BI 0114
C2H5OCOCOOC2H5
Diethyl ester of oxalic acid
Diethyl ester, oxalic acid
Diethylester kyseliny stavelove
diethylesterkyselinystavelove
dlethyloxalate
Oxalic ether
oxalicether
Diethyl oxate
DIETHYL OXALATE, STANDARD FOR GC
DIETHYL OXALATE, 99+%
DiethyloxalateForSynthesis
diethyl ethaneioate | [EINECS(EC#)]
202-464-1 | [Molecular Formula]
C6H10O4 | [MDL Number]
MFCD00009119 | [Molecular Weight]
146.14 | [MOL File]
95-92-1.mol |
| Chemical Properties | Back Directory | [Appearance]
colourless liquid | [Melting point ]
-41 °C (lit.) | [Boiling point ]
185 °C (lit.) | [density ]
1.076 g/mL at 25 °C(lit.)
| [vapor density ]
5.03 (vs air)
| [vapor pressure ]
1 mm Hg ( 47 °C)
| [refractive index ]
n20/D 1.410(lit.)
| [Fp ]
168 °F
| [storage temp. ]
Store below +30°C. | [solubility ]
Miscible with alcohols, ether and other common organic solvents. | [form ]
Liquid | [color ]
Clear | [Stability:]
Stable, but moisture sensitive. Incompatible with strong oxidizing agents. | [explosive limit]
0.42-2.67%(V) | [Water Solubility ]
MAY DECOMPOSE | [Sensitive ]
Moisture Sensitive | [Merck ]
14,3125 | [BRN ]
606350 | [Dielectric constant]
8.2(21℃) | [InChIKey]
WYACBZDAHNBPPB-UHFFFAOYSA-N | [LogP]
0.560 | [CAS DataBase Reference]
95-92-1(CAS DataBase Reference) | [NIST Chemistry Reference]
Ethanedioic acid, diethyl ester(95-92-1) | [EPA Substance Registry System]
95-92-1(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed.
R36:Irritating to the eyes. | [Safety Statements ]
S23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) . | [RIDADR ]
UN 2525 6.1/PG 3
| [WGK Germany ]
1
| [RTECS ]
RO2800000
| [Autoignition Temperature]
410 °C | [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
29171900 | [Safety Profile]
Poison by ingestion.
Flammable liquid when exposed to heat or
flame; can react with oxidzing materials. To
fight fire, use foam, CO2, dry chemical.
When heated to decomposition it emits
acrid smoke and fumes. See also
OXALATES and ESTERS. | [Hazardous Substances Data]
95-92-1(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 400 mg/kg |
| Hazard Information | Back Directory | [General Description]
A colorless liquid. Flash point 168°F. Slightly denser than water and insoluble in water. Hence sinks in water. May irritate skin and mucous membranes; may be mildly toxic by ingestion; may emit irritating fumes in a fire. Vapors are much heavier than air. Used as a solvent for plastics and in the manufacture of perfumes and pharmaceuticals. | [Reactivity Profile]
ETHYL OXALATE(95-92-1) is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides | [Air & Water Reactions]
Insoluble in water. | [Hazard]
Toxic by ingestion, strong irritant to skin
and mucous membranes. | [Health Hazard]
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Contact with molten substance may cause severe burns to skin and eyes. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. | [Fire Hazard]
Combustible material: may burn but does not ignite readily. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water. | [Chemical Properties]
colourless liquid | [Uses]
manufacture of phenobarbital, ethylbenzyl malonate, triethylamine, and similar chemicals, plastics, dyestuff intermediates. Solvent for cellulose esters, perfumes. | [Application]
Diethyl oxalate has the general properties of esters. It absorbs moisture in the air and decomposes slowly. It reacts with ammonia to form amide compounds and condenses with acetone to ethyl pyruvate. It is mainly used in the pharmaceutical industry. It is an intermediate of azathioprine, peripheral sulfanilamide, carboxyphenyllipase penicillin, ethoxybenzylpenicillin, chloroquine lactate, thiabendazole, sulfamethoxazole and other drugs. It can be used as an intermediate of plastics, dyes and other products, and as a solvent of cellulose and spices.
Diethyl acetate is often used as the substrate of nucleophilic reagent α,γ- Dicarbonyl esters, ketone compounds, synthesis of heterocyclic compounds, etc. synthesis α,γ- Dicarbonyl esters can be formed by nucleophilic substitution reaction between ketones and diethyl oxalate under alkaline conditions α,γ- Dicarbonyl ester (formula 1). The dicarbonyl ester often exists in enol structure and can be used to synthesize heterocyclic compounds (formula 2)
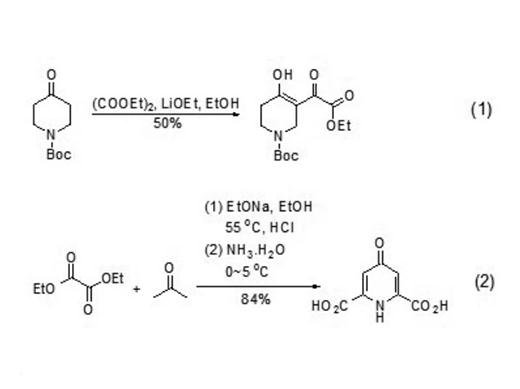 | [Production Methods]
Diethyl oxalate is produced via esterification of ethanol and
oxalic acid. It is a preferred solvent for cellulose acetate and
nitrate. |
|
|