| Identification | Back Directory | [Name]
2-DI-TERT-BUTYLPHOSPHINO-2',4',6'-TRIISOPROPYLBIPHENYL | [CAS]
564483-19-8 | [Synonyms]
2&lsquo
tBuXPhos
t-Bu XPhos
tBuXPhos 97%
98% tBuXPhos
di-t-Bu-XPhos
t-butyl-Xphos
TERT-BUTYL X-PHOS
2-Di-t-butylphosphino-2',4',6'
-DI-Tert-Butylphosphino-2,4,6-Triisopropylbiphenyl
2-di-tert-butylphosphino-2',4',6'-triisopropylbiph
2-Di-tert-butylphosphino-2',4',6'-trisopropylbinphenyl
2-DI-TERT-BUTYLPHOSPHINO-2',4',6'-TRIISOPROPYLBIPHENYL
2-DI-T-BUTYLPHOSPHINO-2',4',6'-TRI-I-PROPYL-1,1'-BIPHENYL
2-Di-t-butylphosphiNA-2',4',6'-tri-i-propyl-1,1'-biphenyl
Di-tert-butyl(2',4',6'-triisopropylbiphenyl-2-yl)phosphine
2-Di-tert-butylphosphino-2',4',6'-triisopropylbiphenyl ,98%
2-DI-TERT-BUTYLPHOSPHINO-2',4', 6'-TRI-I-PROPYL-1,1'-BIPHENYL
2-Di-t-butylphosphino-2',4',6'-tri-i-propyl-1,1'-biphenyl,min.98%
Di-tert-butyl(2',4',6'-triisopropyl-[1,1'-biphenyl]-2-yl)phosphine
2-Di-t-butylphosphino-2',4',6'-tri-i-propyl-1,1'-biphenyl,98% tBuXPhos
2-DI-T-BUTYLPHOSPHINO-2'',4'',6''-TRI-I-PROPYL-1,1''-BIPHENYL, MIN. 98%
2-Di-tert-butylphosphino-2′,4′,6′-triisopropylbiphenyl,tert-Butyl XPhos
2-Di-t-butylphosphino-2',4',6'-tri-i-propyl-1,1'-biphenyl, t-butylXPhos
2-Di-t-butylphosphino-2',4',6'-tri-i-propyl-1,1'-biphenyl,min.98%t-butylXPhos
2-Di-t-butylphosphino-2',4',6'-tri-i-propyl-1,1'-biphenyl, Min. 98% t-Bu-X-PHOS
Bis(1,1-dimethylethyl)[2',4',6'-tris(1-methylethyl)[1,1'-biphenyl]-2-yl]phosphine
phosphine, bis(1,1-diMethylethyl)[2',4',6'-tris(1-Methylethyl)[1,1'-biphenyl]-2-yl]- | [EINECS(EC#)]
1312995-182-4 | [Molecular Formula]
C29H45P | [MDL Number]
MFCD06411306 | [MOL File]
564483-19-8.mol | [Molecular Weight]
424.64 |
| Questions And Answer | Back Directory | [Uses]
tBuXPhos [2-Di-tert-butylphosphino-2′,4′,6′-triisopropylbiphenyl] is an air-stable, electron-rich biaryl phosphine ligand developed by the Buchwald group to enhance the reactivity of palladium catalysis during cross-coupling reactions. tBuXPhos has been used in the preparation of [tBuXPhosAu(MeCN)]BAr4F, a gold catalyst for the intermolecular [2+2] cycloaddition of terminal arylalkynes with substituted alkenes to form functionalized cyclobutenes with high regioselectivity.
tBuXPhos is a ligand for Pd-catalyzed C-O and C-N bond formation.
It can be used in the following reactions:
? Palladium-catalyzed Tsuji-Trost substitution and cross-coupling of benzylic fluorides.
? Palladium-catalyzed C-N cross-coupling of sulfinamides and aryl halides.
? Palladium-catalyzed rapid methoxylation and deuteriomethoxylation of bromo-chalcones. | [Reactions]
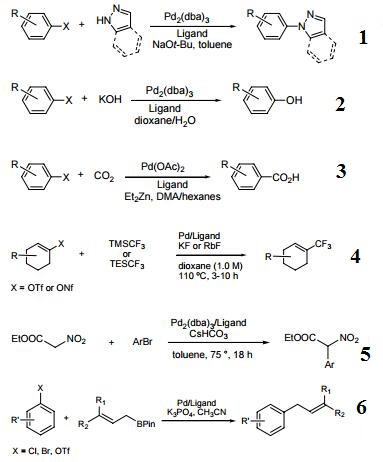
- Effective ligand for the Pd-catalyzed arylation of pyrazoles, indazoles and amino heterocycles.
- Ligand used in the Pd-catalyzed synthesis of phenols from aryl halides and KOH.
- Ligand used in the Pd-catalyzed of benzoic acids from aryl halides and CO2.
- Ligand used in the Pd-catalyzed trifluoromethylation of vinyl sulfonates.
- Ligand used in the Pd-catalyzed arylation of nitroacetates.
- Ligand used in the Pd-catalyzed Suzuki−Miyaura cross-coupling of allylboronates and aryl halides.
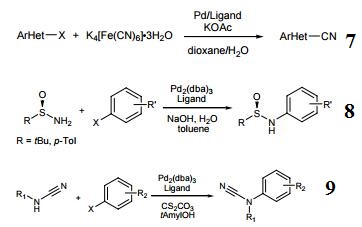
- Ligand used in the Pd-catalyzed cyanation of (hetero)arylchlorides and bromides.
- Ligand used in the Pd-catalyzed C–N cross coupling of sulfinamides and aryl halides.
- Ligand used in the Pd-catalyzed arylation of cyanamides.
|
| Chemical Properties | Back Directory | [Melting point ]
148-151 °C(lit.)
| [Boiling point ]
493.5±45.0 °C(Predicted) | [solubility ]
soluble in Toluene | [form ]
Crystalline Powder | [color ]
White | [InChI]
InChI=1S/C29H45P/c1-19(2)22-17-24(20(3)4)27(25(18-22)21(5)6)23-15-13-14-16-26(23)30(28(7,8)9)29(10,11)12/h13-21H,1-12H3 | [InChIKey]
SACNIGZYDTUHKB-UHFFFAOYSA-N | [SMILES]
P(C(C)(C)C)(C(C)(C)C)C1=CC=CC=C1C1=C(C(C)C)C=C(C(C)C)C=C1C(C)C |
| Hazard Information | Back Directory | [Chemical Properties]
White to pale yellow | [Uses]
Effective ligand for the Pd-catalyzed arylation of pyrazoles, indazoles and amino heterocycles | [Uses]
suzuki reaction | [General Description]
tBuXPhos [2-Di-tert-butylphosphino-2′,4′,6′-triisopropylbiphenyl] is an air-stable, electron-rich biaryl phosphine ligand developed by the Buchwald group to enhance the reactivity of palladium catalysis during cross-coupling reactions. | [reaction suitability]
reagent type: ligand |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Ethyl acetate-->Tetrahydrofuran-->Dichloromethane-->Government regulation-->Benzene-->Aluminum chloride-->Carbon tetrachloride-->Iron-->Magnesium-->Phosphorus trichloride-->Copper(I) chloride-->1,2-Dibromoethane-->2-Bromopropane-->2-Chloro-2-methylpropane-->1,2-Dibromobenzene | [Preparation Products]
1-Butanone, 1-(4-fluorophenyl)-4-[(6bS,10aR)-2,3,6b,9,10,10a-hexahydro-3-methyl-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxalin-8(7H)-yl]--->tBuXPhos-Pd-G3 |
|
|