| Identification | More | [Name]
2-Dicyclohexylphosphino-2'-(N,N-dimethylamino)biphenyl | [CAS]
213697-53-1 | [Synonyms]
2-(DICYCLOHEXYLPHOSPHINO)-2'-(DIMETHYLAMINO)BIPHENYL
2-DICYCLOHEXYLPHOSPHINO-2'-(N,N-DIMETHYLAMINO)BIPHENYL
DAVEPHOS
2-DICYCLOHEXYLPHOSPHINO-2'-(N,N-DIMETHY&
2-Dicyclohexylphosphino-2'-(N,
2-(Dicyclohexylphosphino)-2'-(N,N-dimethylamino)biphenyl,98%DavePhos
2-Dicyclohexylphosphino-2'-(N,N-dimethylamino)-
[1,1''-BIPHENYL]-2-AMINE, 2''-(DICYCLOHEXYLPHOSPHINO)-N,N-DIMETHYL-
2-Dicgclohexylphosphino-2’-(N,N-dimethylamino)biphenyl
Davep
2-Dicyclohexylphosphino-2'-(dimethylamino)biphenyl
2-(N,N-Dimethylamino)-2-(dicyclohexylphosphino)biphenyl | [EINECS(EC#)]
606-749-5 | [Molecular Formula]
C26H36NP | [MDL Number]
MFCD02183572 | [Molecular Weight]
393.54 | [MOL File]
213697-53-1.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light yellow crystals or | [Melting point ]
121-124 °C(lit.)
| [Boiling point ]
539.6±43.0 °C(Predicted) | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [form ]
crystal | [pka]
5.04±0.18(Predicted) | [color ]
white | [Water Solubility ]
Insoluble | [Sensitive ]
Air Sensitive | [Usage]
Ligand used in the palladium-catalyzed Suzuki coupling and amination of unactivated aryl chlorides. | [Detection Methods]
GC | [InChIKey]
ZEMZPXWZVTUONV-UHFFFAOYSA-N | [CAS DataBase Reference]
213697-53-1(CAS DataBase Reference) | [Storage Precautions]
Air sensitive;Store under nitrogen |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,Xn | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R22:Harmful if swallowed. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [WGK Germany ]
3
| [TSCA ]
No | [HazardClass ]
AIR SENSITIVE | [HS Code ]
29310099 |
| Hazard Information | Back Directory | [Description]
2-Dicyclohexylphosphino-2'-(N,N-dimethylamino)biphenyl is a potent inhibitor of the enzyme bromodomain. Bromodomain has been implicated in cancer and other diseases. It binds to the bromodomain in a covalent manner, blocking the ATP site and inhibiting bromodomain function. This inhibition has been shown to be selective for bromodomains over other domains. | [Chemical Properties]
white to light yellow crystals or | [Uses]
2-Dicyclohexylphosphino-2'-(N,N-dimethylamino)biphenyl can act as ligand used in the palladium-catalyzed Suzuki coupling and amination of unactivated aryl chlorides.
| [Uses]
Effective ligand in the Pd-catalyzed arylation of ester enolates | [Uses]
suzuki reaction | [Application]
2-Dicyclohexylphosphino-2'-(N,N-dimethylamino)biphenylacts as a growth regulator by binding to Toll-like receptor 4 (TLR4), which is important in cytokine signaling. It also inhibits allyl carbonate production and cross coupling reactions between aryl chlorides and nitrogen atoms or amines. It has an inhibitory effect on depression, but this effect is not related to its inhibitory effect on bromodomains. |
| Questions And Answer | Back Directory | [Reaction]
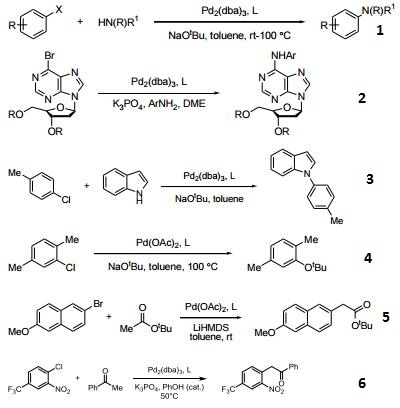
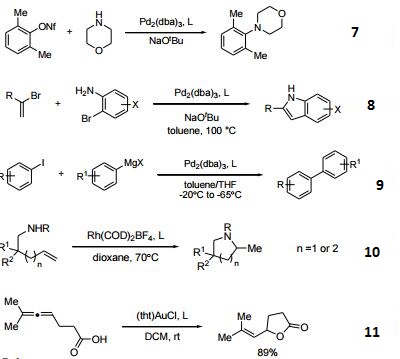
- Ligand used in the Pd-catalyzed Suzuki coupling and animation of unactivated aryl chlorides. The reactions generally occur at room temperature and give high yields of product.
- Ligand used in Pd-catalyzed C-N bond formation. A general synthesis of N6-aryl-2'deoxyadenosine analogues.
- Ligand used in Pd-catalyzed N-arylation of indoles.
- Ligand used in Pd-catalyzed synthesis of aryl-tert-butyl ethers.
- Effective ligand in the Pd-catalyzed arylation of ester enolates.
- Ligand employed in arylation of ketone enolates using ortho-halo nitrobenezenes.
- Ligand employed in the amination of aryl nonaflates using Pd catalysts.
- Ligand used for cascade alkenyl amination/Heck reaction for the synthesis of indoles.
- Ligand used in Pd-catalyzed Kumada-Corriu cross coupling at low temperatures.
- Ligand used in Rh-catalyzed intramolecular hydroamination of unactivated terminal and internal alkenes with primary and secondary amines.
- Ligand used in Au-catalyzed cycloisomerization of allenes.
|
|
|