| Identification | Back Directory | [Name]
2-Dicyclohexylphosphino-2',6'-dimethoxybiphenyl | [CAS]
657408-07-6 | [Synonyms]
6´
98% SPhos
SPhos 97%
SPhos, >=98%
REF DUPL: S-PHOS
-diMethoxybiphenyl
S-Phos, ChemDose(R) tablets
S-Phos, ChemDose? tablets
2-Dicyclohexylphosphino-2',6'-
2-Dicyclohexylphosphino-2´
2-Dicyclohexylphosphino-2',6'-dimethoxybipheny
2-Dicyclohexylphosphino-2', 6-diMethoxybihenyl
2-DicyclohexylphosphiNA-2',6'-diMethoxybiphenyl
2-Dicyclohexylphosphino-2',6'-dimethoxybiphenyl
2-(Dicyclohexylphosphanyl)-2',6'-dimethoxybiphenyl
Dicyclohexyl(2',6'-dimethoxybiphenyl-2-yl)phosphine
2-Dicyclohexylphosphino-2',6'-dimethoxybiphenyl, 98+%
Dicyclohexyl-[2-(2,6-dimethoxyphenyl)phenyl]phosphane
2-Dicyclohexylphosphino-2',6'-dimethoxybiphenyl, min. 98%
2-Dicyclohexylphosphino-2',6'-diMethoxy-1,1'-biphenyl ,98%
Dicyclohexyl(2',6'-diMethoxy-[1,1'-biphenyl]-2-yl)phosphine
Phosphine,dicyclohexyl(2',6'-dimethoxy[1,1'-biphenyl]-2-yl)-
S-PHOS, 2-Dicyclohexylphosphino-2,6-di-Methyloxy-1,1-biphenyl
2-Dicyclohexylphosphino-2’,6’-dimethoxy-1,1’-biphenyl (S-Phos)
2-Dicyclohexylphosphino-2',6'-dimethoxy-1,1'-biphenyl, min. 98%
2-Dicyclohexylphosphino-2',6'-diMethoxy-1,1'-biphenyl,98% Sphos
2 - dicyclohexyl phosphine - 2 ', 6 '- diMethoxy - 1, 1' - two PCB
dicyclohexyl({2-[(2,6-dimethoxyphenyl)methyl]phenyl}methyl)phosphane
2-Dicyclohexylphosphino-2',6'-diMethoxybiphenyl, 6'-diMethoxybiphenyl
2-DICYCLOHEXYLPHOSPHINO-2',6'-DIMETHOXY-1,1'-BIPHENYL, MIN. 98% S-PHOS
S-Phos impregnated tablets, ChemDose(R), S-Phos tablets, 2-Dicyclohexylphosphino-2μ,6μ-dimethoxybiphenyl impregnated tablets | [EINECS(EC#)]
2017-001-1 | [Molecular Formula]
C26H35O2P | [MDL Number]
MFCD05861611 | [MOL File]
657408-07-6.mol | [Molecular Weight]
410.53 |
| Questions And Answer | Back Directory | [Uses]
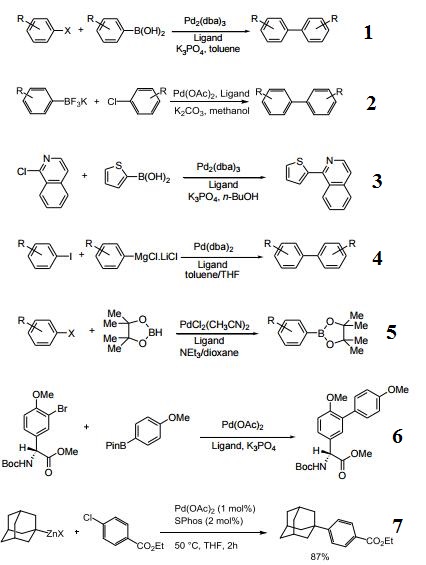
SPhos [2-Dicyclohexylphosphino-2′,6′-dimethoxybiphenyl] is an air-stable, electron-rich biaryl phosphine ligand developed by the Buchwald group to enhance the reactivity of palladium catalysis during cross-coupling reactions.
SPhos may be used as a ligand in the following processes:
? Palladium catalyzed Suzuki-Miyaura cross-coupling reaction between Boc-protected aminomethyltrifluoroborate and aryl chlorides or hetaryl chlorides to form the corresponding aminomethylarenes.? Palladium catalyzed Suzuki-Miyaura cross-coupling reaction between 4-methyl-substituted piperidinylzinc reagent and different aryl or heteroaryl iodides to form various substituted piperidines.? Intramolecular Suzuki-Miyaura coupling to form the 18-membered macrocyclic ring during the multi-step synthesis of riccardin C.
Utilized in conjunction with palladium to form a highly active catalyst for C-N bond formation.
Highly universal ligand for Suzuki-Miyaura coupling; aryl chlorides, hindered biaryls, heterobiaryls. | [Reactions]
- Ligand/palladium catalyst for general Suzuki-Miyaura cross-coupling reactions.
- Ligand/palladium catalyst for the Suzuki-Miyaura coupling of aryltrifluoroborates with aryl chlorides.
- Ligand/palladium catalyst for the Suzuki-Miyaura reaction of heteroaryl halides and heteroaryl boronic acids and esters.
- Ligand/palladium catalyst for the Kumada-Corriu cross-coupling reaction.
- Ligand/palladium catalyst for the borylation of aryl halides with pinacol borane.
- Suzuki couplings involving amino acids. Synthesis of biaryl derivatives of 4-hydroxyphenyl glycine, tyrosine and tryptophan.
- Synthesis of substituted adamantylzinc reagents using Mg-insertion in the presence of zinc chloride.
- Highly efficient catalyst for the palladium-catalyzed Suzuki-Miyura reaction of heteroaryl halides and heteroaryl boronic acids
- and esters.
|
| Chemical Properties | Back Directory | [Melting point ]
164-166°C | [Boiling point ]
513.3±40.0 °C(Predicted) | [storage temp. ]
Refrigerated. | [solubility ]
soluble in Chloroform | [form ]
crystal | [color ]
white | [Sensitive ]
Air Sensitive | [InChIKey]
VNFWTIYUKDMAOP-UHFFFAOYSA-N |
| Hazard Information | Back Directory | [Chemical Properties]
White solid | [Uses]
suzuki reaction | [General Description]
SPhos [2-Dicyclohexylphosphino-2′, 6′-dimethoxybiphenyl] is an air-stable, electron-rich biaryl phosphine ligand developed by the Buchwald group to enhance the reactivity of palladium catalysis during cross-coupling reactions. | [reaction suitability]
reagent type: catalyst
reaction type: Cross Couplings |
|
|