| Identification | More | [Name]
X-PHOS | [CAS]
564483-18-7 | [Synonyms]
2-(DICYCLOHEXYLPHOSPHINO)-2',4',6'-TRI-I-PROPYL-1,1'-BIPHENYL
2-DICYCLOHEXYLPHOSPHINO-2',4',6'-TRIISO-PROPYL-1,1'-BIPHENYL
2-DICYCLOHEXYLPHOSPHINO-2',4',6'-TRIISOPROPYLBIPHENYL
5-BROMO-4-CHLORO-3-INDOLYL PHOSPHATE
X-PHOS
2-Dicyclohexylphosphino-2',4',6'-tri-i-propyl-1,1'-biphenyl, X-PHOS
2-(Dicyclohexylphosphino)-2',4',6'-tri-i-propyl-1,1'-biphenyl,min.98%X-Phos
2-Dicyclohexylphosphino-2',4',6'-triisopropylbiphenyl, 98+%
ChemDose(R), X-Phos impregnated tablets, 2-Dicyclohexylphosphino-2μ,4μ,6μ-triisopropylbiphenyl impregnated tablets
X-Phos, ChemDose(R) tablets | [EINECS(EC#)]
611-387-6 | [Molecular Formula]
C33H49P | [MDL Number]
MFCD04117682 | [Molecular Weight]
476.72 | [MOL File]
564483-18-7.mol |
| Chemical Properties | Back Directory | [Appearance]
White crystalline powder | [Melting point ]
187-190 °C(lit.)
| [Boiling point ]
569.8±50.0 °C(Predicted) | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
Benzene (Slightly) | [form ]
Powder | [color ]
white | [Sensitive ]
Air Sensitive | [Stability:]
Air Sensitive | [InChIKey]
UGOMMVLRQDMAQQ-UHFFFAOYSA-N | [CAS DataBase Reference]
564483-18-7(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin .
R22:Harmful if swallowed.
R40:Limited evidence of a carcinogenic effect. | [Safety Statements ]
S37/39:Wear suitable gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37:Wear suitable protective clothing and gloves . | [WGK Germany ]
3
| [TSCA ]
No | [HS Code ]
29319099 |
| Hazard Information | Back Directory | [Chemical Properties]
White crystalline powder | [Uses]
Exceptional ligands for Pd-catalyzed amination and amidation of aryl sulfonates. XPHOS is used as a regent in the synthesis of novel [1,2,4]triazolo[4,3-a]pyrazine derivatives as potential selective c-met inhibitors with improved pharmacokinetic properties. | [Uses]
suzuki reaction | [General Description]
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Click here for more information. |
| Questions And Answer | Back Directory | [Reaction]
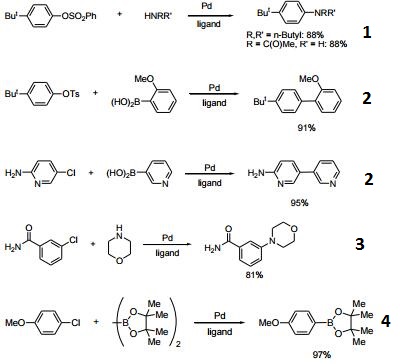
- Exceptional ligands for Pd-catalyzed amination and amidation of aryl sulfonates.
- Ligand used for the Pd-catalyzed Suzuki-Miyaura coupling reaction and carbonyl enolate coupling.
- Ligand used for the chemoselective amination of aryl chlorides.
- Ligand used for the Pd-catalyzed borylation of aryl chlorides, for the formation of trifluoroborates.
- Ligand used for the Pd-catalyzed amination of vinyl halides and triflates.
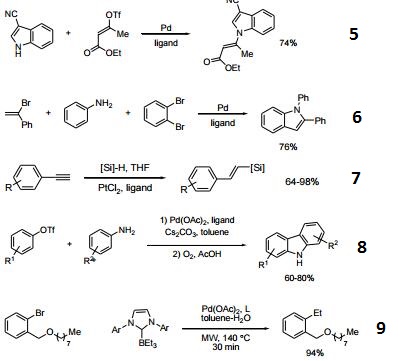
- Ligand used for the Pd-catalyzed three-component synthesis of indoles.
- Ligand used for the Pt-catalyzed regioselective hydrosilylation of functionalized terminal arylalkynes.
- Ligand used for the Pd-catalyzed synthesis of carbazoles.
- Ligand used for the Pd-catalyzed Suzuki-Miyaura coupling of aryl chloride and NHC-boranes.
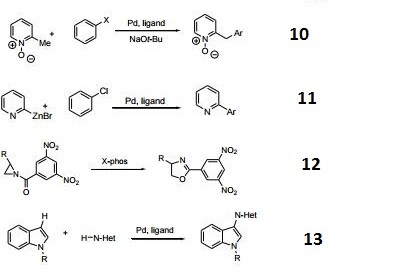
- Ligand used for the direct arylation of picoline N-oxide.
- Ligand used for the Negishi coupling of 2-heterocyclic organozinc reagents.
- Catalyst for a phosphine-catalyzed Heine reaction.
- Ligand used for the palladium-catalyzed oxidative coupling of indoles and heteroarenes.
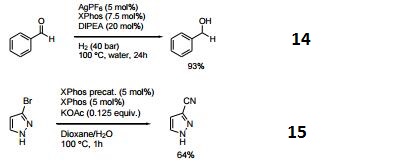
- Ligand used for the silver-catalyzed hydrogenation of aldehydes.
- Ligand used for the palladium-catalyzed cyanation of heterycyclic halides.
|
|
|